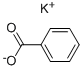
Synthesis and Applications of Potassium Benzoate
General description
Sodium benzoate (E211) and potassium sorbate (E202) are invaluable preservatives. European regulations also allow for the use of benzoic acid (E210), potassium benzoate (E212), calcium benzoate (E213) and derivatives of p-hydroxybenzoic acid. These compounds have been used for large-scale food, beverage, and cosmetic preservation over many decades, a number of bodies—amongst them the U.S. Food and Drug Administration and the European Union—having formulated clear legal provisions regulating their use. In the body, benzoate readily undergoes conjugation with glycine in the liver and kidney, this conversion to hippurate increasing its water solubility in order that it can be efficiently removed from the body by the kidneys. Dietary sorbic acid can be metabolized by the same oxidation pathway as short-chain fatty acids[1].
Synthesis
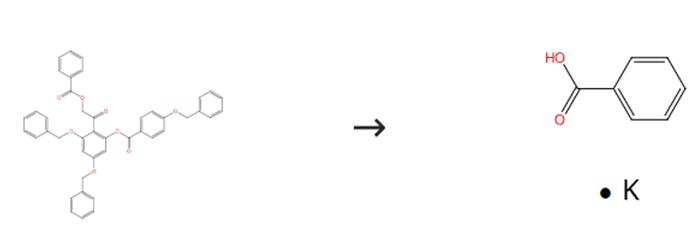
Fig. 1 The synthesis route of Potassium benzoate
Scheme1 Add2-benzoyloxy-2'-(4''-(benzyloxy)benzoyloxy)-4',6'-dibenzyloxyacetophenone (1.44 mmol) to a suspension of sodium hydride (230 mg, 5.76 mmol) in dry THF (20 mL) in THF (10 mL). Reflux the mixture for 90 minutes while stirring. Pour the cooled mixture into a mixture of ice containing concentrated HCl (2 mL). Extract the reaction mixture with CH2Cl2(3 × 100 mL). Remove the solvent. Dissolve the resulting residue in AcOH (11 mL). Add AcONa (181 mg) to the reaction mixture. Stir the reaction mixture at 100°C for 12 hours. Cool the mixture to room temperature. Dilute the reaction mixture with CH2Cl2. Wash the mixture with water and aqueous NaHCO3.Concentrate on the organic phase. Dissolve the reaction mixture in MeOH/CH2Cl2(8 mL, v/v 1:1). Stir the reaction mixture for 10 hours in the presence of a catalytic amount of NaOMe at room temperature. Neutralize the mixture with Dowex 50W-X 8 (H+) resin. Filter the resins.Concentrate the filtrate in vacuo.Purify the residue by silica gel column chromatography (acetone-petroleum ether-CH2Cl2, 1:20:8). The synthesis route is shown in Fig. 1.
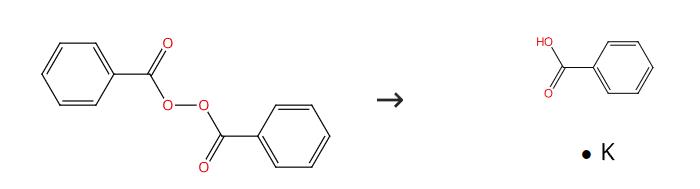
Fig. 2 The synthesis route of Potassium benzoate
Scheme2 Place a weighed portion of KOH into the cell. Add a fixed volume of DMF to the mixture. Allow the system to stand for 30 minutes at 328 K. Add the peroxide to the mixture. Monitor the process kinetics iodometrically. As a kinetic parameter, we used the initial decomposition rate ν0. Determine liquid reaction product by gas chromatography on a Chrom-5 instrument (flame-ionization detector; 3000 x3 mm glass column; stationary phase SE-30; carrier gas argon; temperature-programmed mode, 323-523 K; heating rate 8 deg min-1). Process the resulting chromatograms using an internal reference. Carry out the alkylation of intermediate sodium perbenzoate at 290 K using the same procedure as in studying the decomposition kinetics. Introduce the alkylating agent 15 minutes after the addition of the peroxide. Monitor the process by the consumption of available oxygen using the iodometric method and also by the accumulation of butyl benzoate using GC. Since peroxy compounds are unstable under GC conditions, reduce the peroxy compound with triphenylphosphine before analysis. The synthesis route is shown in Fig. 2.
Applications
In the food industry, potassium benzoate is predominantly used to preserve acidic foods and beverages, where it effectively prevents microbial growth and extends shelf life. It is particularly valued in products such as soft drinks, fruit juices, pickles, and condiments like ketchup and salad dressings. These items, often characterized by their acidic pH, create an environment where potassium benzoate acts as an antimicrobial agent, thereby ensuring the safety and longevity of the products. Its use in the beverage sector is especially noteworthy, with soft drinks being one of the largest consumers of this preservative, due to its ability to maintain product quality and prevent spoilage.
Using chemical substances to prevent or delay food spoilage is partly due to the great success of these compounds in the treatment of diseases in humans, animals and plants. Although a large number of chemical compounds are effective food preservatives, yet because of strict laws on food safety that have been adopted by the FDA and to a lower extent due to the fact that, in vitro, all compounds show antimicrobial effects, adding these compounds to some food products has no effect and only a few of them are permitted for use in food products.
Nowadays, processed foods make up 75 percent of the diet of western societies. Sodium benzoate is one of the synthetic additives that are widely used in the food industry and is generally recognized as safe (GRAS). Sodium benzoate is a salt of benzoic acid, which is used as an important preservative in the food industry against bacteria, fungi and yeast with the natural pH of 4.5. Also, this substance can be used in pharmaceutical and cosmetics industries[2].
References
[1]Piper P W. Potential safety issues surrounding the use of benzoate preservatives[J]. Beverages, 2018, 4(2): 33.
[2] Shahmohammadi M, Javadi M, Nassiri-Asl M. An overview on the effects of sodium benzoate as a preservative in food products[J]. Biotechnology and Health Sciences, 2016, 3(3): 7-11.
You may like
Related articles And Qustion
Lastest Price from Potassium benzoate manufacturers

US $1.00/KG2025-04-21
- CAS:
- 582-25-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $99.00-35.00/kg2025-04-15
- CAS:
- 582-25-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000 ton