Dicyclohexylcarbodiimide(DCC) vs 4-(Dimethylamino)pyridine(DMAP): the difference between the two
What is Dicyclohexylcarbodiimide(DCC)?
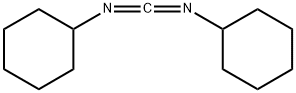
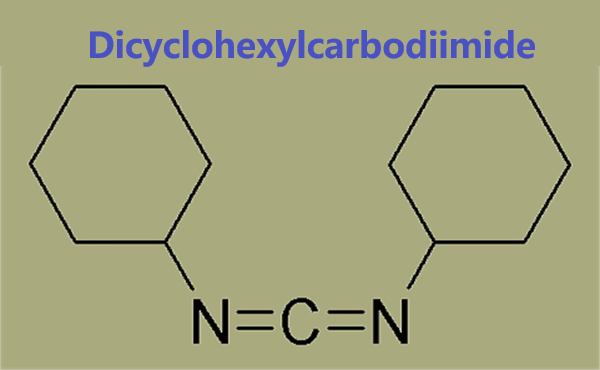
DCC is the acronym for N,N′-dicyclohexylcarbodiimide. In peptide synthesis, it is utilized for the activation of carboxyl group. The structure of DCC has been analyzed based on IR and Raman spectral data. It mediates the esterification of poly(vinyl alcohol) with free levulinic acid to form poly(vinyl alcohol-co-vinyl levulinate).
What is 4-(Dimethylamino)pyridine(DMAP)?
4-(Dimethylamino)pyridine (DMAP) is a highly versatile nucleophilic catalyst for acylation reactions and esterifications. It is also employed in various organic transformations like Baylis-Hillman reaction, Dakin-West reaction, protection of amines, C-acylations, silylations, applications in natural products chemistry, and many others.
Dicyclohexylcarbodiimide(DCC) vs 4-(Dimethylamino)pyridine(DMAP)
Dicyclohexylcarbodiimide is used in industry as a stabilizing agent, coupling agent, and condensing agent. It has widespread use during protein synthesis in the recombinant DNA industry and in the synthesis of polypeptides in the chemical and pharmaceutical industries. Increasing use in field of bioenergetics; stabilizing agent in elastomers, natural rubber, and many types of polyolefins, polyesters, resins, fibers, cellulose esters, etc.
DCC is used as a carboxylate activation reagent for peptide synthesis. DCC has been used in the preparation of titanate nanotubes-phthalocyanine(TiONts-Pc) nanohybrids and 7-((tert-butyldimethylsilyl)oxy)hepta-2,4-diyn-1-yl propynoate. DCC may be used to promote the esterification of 7-((tert-butyldimethylsilyl)oxy)hepta-2,4-diyn-1-ol with propiolic acid to form 7-((tert-butyldimethylsilyl)oxy)hepta-2,4-diyn-1-yl propynoate.
It can also used to synthesize:
1,3-Thiazetedine derivatives via [2+2] cycloaddition with 2-phenylethenyl- and 2-(4-nitrophenyl)ethenyl isothiocyanates.
1,3,5-Oxadiazine-4-thiones via [4+2] cycloaddition with benzoyl isothiocyanates.
Sterically hindered 1,3,4-oxadiazole derivatives by reacting with (N-isocyanimino)triphenylphosphorane in the presence of aromatic (or heteroaromatic) carboxylic acids.
However, 4-(Dimethylamino)pyridine can be used as a catalyst:
To synthesize 3,5-disubstituted 2,6-dicyanoaniline by reacting malononitrile, aldehydes, and β-nitroolefins.
For the acylation of alcohols with acid anhydrides under auxiliary base- and solvent-free conditions to synthesize corresponding esters.
In Baylis-Hillman reaction to form carbon-carbon bond by the coupling of an activated alkene with an aldehyde or ketone.
Although both DCC and DMAP are used in chemical synthesis, there are differences in their applications. DCC is mainly used as a carboxylate activation reagent for peptide synthesis, as a coupling agent or as a stabiliser. DMAP, on the other hand, is an efficient catalyst for acylation reactions and is commonly used in acylation and esterification reactions.
You may like
Related articles And Qustion
See also
Lastest Price from Dicyclohexylcarbodiimide manufacturers

US $10.00/kg2025-11-12
- CAS:
- 538-75-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200tons

US $0.00/Kg/Drum2025-04-21
- CAS:
- 538-75-0
- Min. Order:
- 1KG
- Purity:
- 99%GC
- Supply Ability:
- 10 TONS