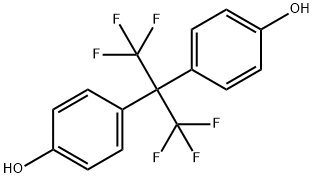
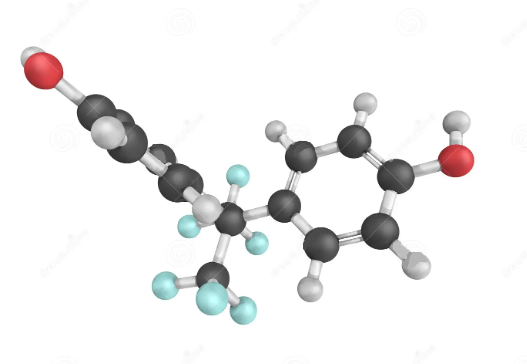
Synthesis and Application of Hexafluorobisphenol A
General description
Hexafluorobisphenol A (Bisphenol AF) is a fluorinated organic compound related to bisphenol A in which the two methyl groups are replaced with trifluoromethyl groups. Whereas bisphenol A binds with human estrogen-related receptor gamma (ERR-γ), Hexafluorobisphenol A all but ignores ERR-γ. Instead, Hexafluorobisphenol A activates ERR-α and binds to and disables ERR-β.
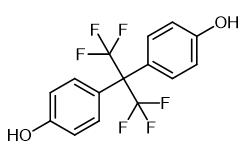
Fig. 1 The structure of Hexafluorobisphenol A.
Physicochemical property
Hexafluorobisphenol A is a white to off-white powder with a melting point of 160-163 °C. It has a boiling point of 400°C and a density of 1.447g/cm3.
Synthetic routes
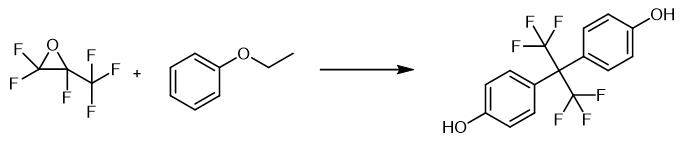
Fig. 2 The synthetic method 1 of Hexafluorobisphenol A.
Fluorine containing hydrogen chloride is fed into the first gas holder, hexafluoride propylene oxide and nitrogen are mixed into the second gas holder according to the mass ratio of 1:2, and phenylpropyl ether and nitrogen are mixed into the third gas holder according to the mass ratio of 1:3, preheating and gasification; After that, hexafluoride propylene oxide mixed with nitrogen was measured by hexafluoride propylene oxide (3.3 kg/min), phenylpropyl ether (11 kg/min), and hydrogen chloride containing fluorine (1.8 kg/min) were measured by phenylpropyl ether (1.8 kg/min) and reacted in a tubular reactor with the reaction temperature of 80 C and the reaction pressure of 1kg/cm2 The reaction time was 3 min, and the reaction material was directly separated into the condensation separation column, and the bisphenol AF product was prepared. The HPLC purity was 99.7%, the yield was 98.3%, and the conversion rate was 99.7% [1].
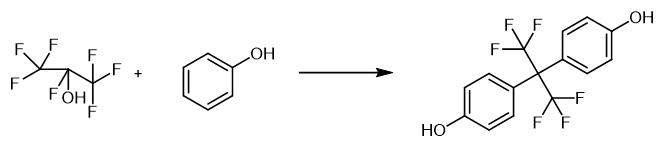
Fig. 3 The synthetic method 2 of Hexafluorobisphenol A.
Charge 188 g (2 mol) of phenol and 375 g of the distillate consisting of 160 g (0.96 mol) of hexafluoroacetone and 215 g (10.75 mol) of HF to a 1 L stainless-steel autoclave. Agitate and heat the reaction mixture in an oil bath up to 110 °C (0.95 MPa). Keep the reaction mixture for 6 h. Pour the reaction mixture into crashed ice, and collect the precipitates. Filter, wash with water and dry to obtain the hexafluorobisphenol. 1H NMR (CD3OD): δ 4.88 (2H, s, OH), 6.76 (2H, d, J = 8.5 Hz, Ph), 7.16 (2H, d, J = 8.5 Hz, Ph). 13C NMR (CD3OD): δ 64.9 (sept,J = 25.2 Hz, (CF3)2C), 115.9, 125.5, 132.6, 158.9 (all s, aromatic), 125.9 (q, J = 286.3 Hz,CF3). IR (ATR, germanium): 1516, 1245, 1211, 1171, 1139 cm-1 19F NMR (CD3OD): δ -63.8 (s, CF3). HRMS (FI): M+, found 336.0565. C15H10F6O2 requires 336.0585 Mass Spec (% rel int.): 336 (M+, 21), 267 (100), 227 (9), 199 (15), 197 (12), 169 (6), 99 (28) [2].
Application
Detection of Dimethyl Methylphosphonate Vapor
Hexafluorobisphenol A (6FBPA), as a novel nerve agents sensing molecule, has been successfully attached onto the surface of single-walled carbon nanotubes (SWNTs). The sensing groups have been confirmed by infrared spectroscopy, Raman spectroscopy and X-ray photoelectron spectrometry. The results revealed that the sensing groups had been successfully anchored on the surface of nanotubes. The quantitative determination of the functional groups has also been carried out through characterization by thermogravimetric analysis. Furthermore, the morphology of the resultant SWNT-6FBPA hybrids has been observed by transmission electron microscopy and scanning electron microscopy. Due to the existence of phenolic hydroxyl groups, which can form strong hydrogen-bonding with dimethyl methylphosphonate (DMMP) (simulant of nerve agent sarin), the functionalized SWNTs showed excellent sensitivity and selectivity while the sensing devices have been fabricated [3].
Spectroscopy
Mid-infrared spectroscopy (400-4000 cm(-1)) of Bisphenol "AF" is manifested. Bisphenol "AF" is another variant of Bisphenol "A," besides Bisphenol "S." Bisphenol "A" is being curbed due to its malign effects. Bisphenol "AF" is gradually taking the place of Bisphenol "A." Bisphenol "AF" tends to have malignancy similar to Bisphenol "A." Various molecular signatures of Bisphenol "AF" have been observed by mid-infrared spectroscopy, which include C-H and O-H stretching vibrations. Observed vibrations are analyzed by density functional theory calculations for possible countermeasure. Principal component analysis is used on detected absorption frequencies of Bisphenol "AF" in conjunction with prior reported frequencies of Bisphenol "A" and "S" in the mid-infrared range. As a result of principal component analysis, the list of correlating absorption frequencies of bisphenol "A," "S," and "AF" in the mid-infrared range is populated, showing the role of benzene rings. This correlation may lead to a group of toxic materials and to finding the origin of their toxicity by knowing the nature of correlated vibrations and the related involvement of functional groups and atoms [4].
Bisphenol "AF," a chemically similar replacement substitute for Bisphenol "A" which is a widespread environmental hormone, is studied by Raman spectroscopy (250-3500 cm(-1)). Experimentally observed scattering peaks are illuminated by Density Functional Theory calculations. Principal component analysis is executed on the experimental Raman spectra of Bisphenol "AF" together with spectra of Bisphenol "A" and "S" reported earlier. Eight correlating molecular frequencies of Bisphenol "AF," "A," and "S" are found in contrast to 12 such frequencies of Bisphenol "A" and "S" only. The refined list of correlating frequencies manifests the existence of correlation in bisphenol family and clue toward their grouping, identification, detection, and screening together with mechanism responsible for toxicity [5].
Interaction with human serum albumin
Bisphenol AF (BPAF) was used as a model compound to investigate the binding mechanism between the endocrine disrupting compound and human serum albumin (HSA) using multispectroscopic techniques and molecular modeling method at the protein level. The results indicated that BPAF was indeed bound to HSA and located in the hydrophobic pocket of HSA on subdomain IIA through hydrogen bond and van der Waals interactions. The fluorescence quenching data showed that the binding of BPAF and HSA quenched the intrinsic fluorescence of HSA, and the static quenching constants were acquired [6].
References
[1] He J, Pei W. Preparation method of bisphenol AF[P]. Faming Zhuanli Shenqing, 111233632, 2020.
[2] Hayasaka T, Katsuhara Y, Kume T, et al. HF-mediated equilibrium between fluorinated ketones and the corresponding α-fluoroalcohols[J]. Tetrahedron, 2011, 67(12): 2215-2219.
[3] Wang Y, Wang Z, Hu N, et al. Hexafluorobisphenol a covalently functionalized single-walled carbon nanotubes for detection of dimethyl methylphosphonate vapor[J]. Journal of Nanoscience and Nanotechnology, 2011, 11(6): 4874-4881.
[4] Ullah R, Wang X. Sensi
Related articles And Qustion
See also
Lastest Price from Bisphenol AF manufacturers
US $0.00-0.00/Kg2025-10-27
- CAS:
- 1478-61-1
- Min. Order:
- 1Kg
- Purity:
- ≥99.5%
- Supply Ability:
- 100MT

US $10.00/KG2025-04-21
- CAS:
- 1478-61-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons