The toxicity of Bisphenol AF
Introduction
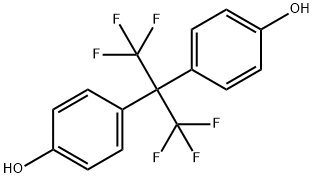
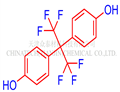
Bisphenol AF (4,4′-(hexafluoroisopropylidene)diphenol) has emerged as an alternative compound to bisphenol A (BPA) and is now being widely utilized in industrial applications. Consequently, bisphenol AF has been detected in various food sources such as vegetables, meat, beverages, and seafood.
Toxicity
Recent studies have demonstrated the detrimental effects of BPA and its structural congeners, including bisphenol AF, bisphenol G, bisphenol M, and bisphenol X, on microalgae species like Chlorella vulgaris and Desmodesmus armatus. Moreover, a toxicity test conducted with a single dose of BPF revealed symptoms like confusion and convulsions following oral administration. Perinatal exposure to bisphenol AF did not induce adverse effects on oxidative damage; however, it led to alterations in the levels of β-glucose and glycogen in the livers of male mouse offspring. Gu et al. investigated bisphenol AF exposure in zebrafish, revealing increased oxidative stress, apoptosis, and suppressed cardiac development gene expression. Beg and Sheikh examined bisphenol AF's impact on the thyroid hormone system in rats, reporting reduced serum T4 and T3 levels, disrupting thyroid hormone signaling[1].
Harm to the environment
Bisphenol AF (BPAF), with a fluorinated form of bisphenol A (BPA), has been in the spotlight as an alternative to BPA in the polycarbonate plastics, epoxy resins, and thermal paper industries as a co-reactant. The BPAF has a high molecular weight (336.23 g/mol) and octanol-water partition coefficient (log Kow = 4.47)[2]. Because of its specific physicochemical properties, it has a long half-life in water (180 days), soil (360 days), and sediment (1620.8 days). When exposed to the environment, BPAF has a higher potential to adsorb to sediments and accumulate in organism tissues than the other bisphenol analogues (BPA, BPS, BPF, and BPB) because it exhibits a high environmental persistence. In recent years, BPAF has been frequently detected in the surface waters (lakes, rivers, and bays) of areas around BPAF manufacturing plants in China. The maximum concentration in the Xitang River was 15,300 ng/L.
Moreover, BPAF was detected at the second highest concentration, after BPA, in surface and drinking water in China. Previous studies have reported that BPAF has higher bioaccumulation potential and is more difficult to biodegrade than BPA. In BPAF, the BPA's CH3 group was replaced by CF3, making it more electronegative and potentially more reactive than BPA. The production and use of BPA are strictly prohibited or regulated in America and the European Union. However, the related regulations and policies for BPAF are still lacking. Compared with BPA, there have been relatively few toxicological studies and potential risk assessments of BPAF.
References:
[1] MANTHAN SHARMA, ROSHAN M. BORKAR* Bisphenol AF Induces Alterations in the Plasma Lipidome of Rats: An Untargeted Lipidomics Approach[C]//1 5. 2023: 300-359. DOI:10.1021/envhealth.3c00108.[2] HI GYU MOON . Assessment of potential environmental and human risks for Bisphenol AF contaminant[J]. Ecotoxicology and Environmental Safety, 2024, 281. DOI:10.1016/j.ecoenv.2024.116598.
You may like
Related articles And Qustion
See also
Lastest Price from Bisphenol AF manufacturers
US $0.00-0.00/Kg2025-10-27
- CAS:
- 1478-61-1
- Min. Order:
- 1Kg
- Purity:
- ≥99.5%
- Supply Ability:
- 100MT

US $10.00/KG2025-04-21
- CAS:
- 1478-61-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons