Is Lithium sulfide an ionic or covalent compound?
Lithium sulfide is an inorganic compound with the formula Li₂S. It crystallizes in the antifluorite motif, described as the salt (Li⁺)₂S²⁻. It forms a solid yellow-white deliquescent powder. In the air, it easily hydrolyses to release hydrogen sulfide.
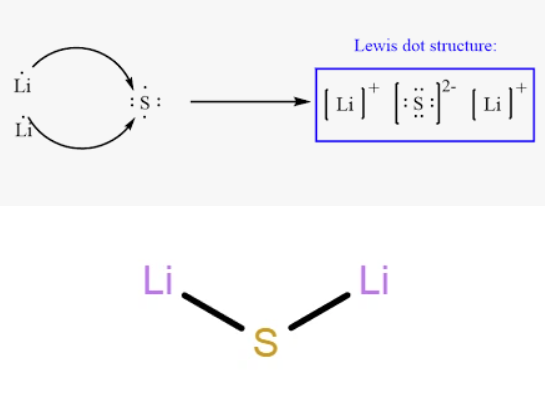
Lithium Sulfide (sulphide) is an ionic compound. In this compound, lithium atoms lose electrons to form positive ions (Li+), while sulfur gains electrons to form negative ions (S2-). These oppositely charged ions are held together by ionic bonds, which are strong electrostatic forces of attraction between the positive and negative ions.
A molecular model of lithium sulfide would depict two spheres representing lithium atoms and one larger sphere representing the sulfur atom. The atoms in lithium sulfide are indeed held together by these ionic bonds, resulting from the transfer of electrons from lithium to sulfur during chemical bonding.
You may like
Related articles And Qustion
See also
Lastest Price from Lithium sulfide manufacturers

US $1.00-2.10/KG2025-08-29
- CAS:
- 12136-58-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20000KG

US $10.00/ASSAYS2025-08-29
- CAS:
- 12136-58-2
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton