Synthesis and Application of D-Penicillamine
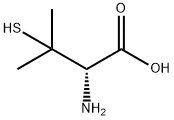
D-Penicillamine (1) is a colorless crystalline powder with a weak odor typical of sulfur-containing amino acids, and a characteristic taste. It is relatively soluble in water but less so in alcohols and almost insoluble in ether, chloroform, carbon tetrachloride, and aliphatic or aromatic hydrocarbons. melts with decomposition at 202—206 º C (Mettler FPmelting point apparatus, starting temperature 195 º C, heating rate 2”/min). Qualitative identification of (1) is based on the reaction.
with iodine solution (decolorized due to oxidation of the SH group), ninhydrin (blue-violet color), phosphortungstic acid (deep blue color), and acetone (colorless precipitate) Quantitative determination is best carried out by titration against mercuric acetate solution in the presence of diphenyl-carbazone as indicator. Traces of impurities, eg. D-penicillamine disulfide may be detected either by TLC or, after silylation, by GLC.
The biochemical properties of D-penicillamine are primarily based on three types of reaction in which the amino and mercapto groups are principally involved:
1) Chelate formation with heavy metal ions;
2) exchange reactions with low molecular and high molecular weight disulfides;
3) condensation with aldehydes.
Synthesis
Both processes produce highly pure D-penicillamine which is then used as the active ingredient in drug manufacturer.[1]
Scheme1: Manufacture of D-Penicillamine can be manufactured either semi-synthetically by degradation of penicillins or completely synthetically via the racemate. Production from penicillins has been considerably improved in the last few years, Purification of (1) is most suitably carried out by conversion into the hydrogen chloride of D-2,2,5,5-tetramethyIthiazoli-dine-4-carboxylic acid (6). This latter product (6) is also known as “D-penicillamine-acetone adduct” or “isopropyli- dene-D-penicillamine” due to the ease with which it can be reconverted into acetone and (1).
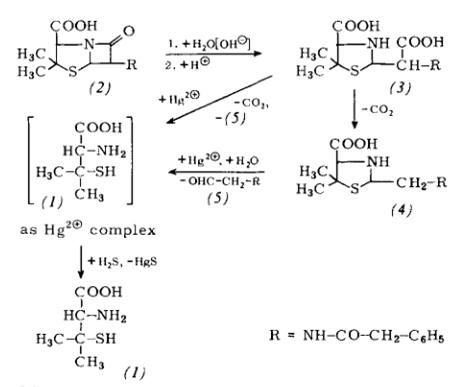

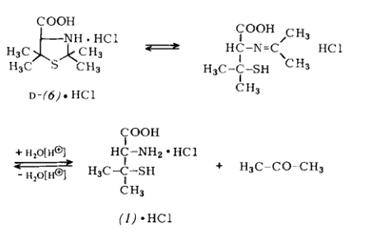
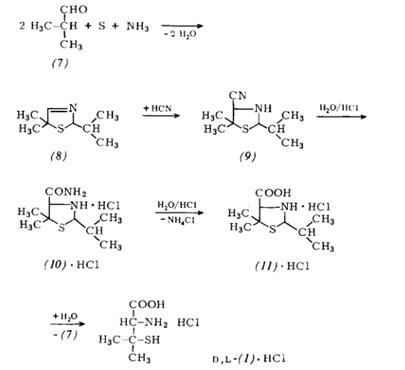
Scheme2: A new process for the manufacture of fully-synthetic D-penicillamine via the racemate and its resolution (“Asinger process”) is now also being carried out industrially.
The simultaneous reaction of sulfur and ammonia with isobutyraldehyde (7) affords 2-isopropyl-5,5-dimethyl-3-thi-azoline (8) in ca. 80% yield if the resulting water is azeotrop-ically removed (see Scheme 2). Benzene, toluene, cyclohexane, chloroform, or preferably an excess of isobutyraldehyde itself can be used as the azeotropic partnerIz61. The 3-thiazoline (8) itself is purified by rectification (b. p. 68℃-70℃/12torr).
Application
D-Penicillamine is an antidote for heavy metals such as copper, mercury and copper. It is also used to treat autoimmune diseases such as hepatolenticular degeneration (Wilson's disease) and rheumatoid arthritis caused by copper deposition in tissues. It is forbidden for patients with penicillin allergy, hematopoietic dysfunction and renal dysfunction
Handling and storage
1.It shall be sealed and stored dry at 4 ℃
2. Store in a cool place. Keep the container closed and store in a dry and ventilated place. Open containers must be carefully resealed and kept upright to prevent leakage.
Reference
[1] "By Wolfgang M. Weigert, Heribert Offermanns, and Paul Scherberich,".
[2]
Lastest Price from D-Penicillamine manufacturers

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 52-67-5
- Min. Order:
- 1KG
- Purity:
- 97%-102%;USP
- Supply Ability:
- 200KGS

US $990.00-800.00/kg2025-04-21
- CAS:
- 52-67-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000