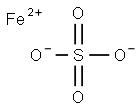Application of Ferrous sulfate
General description
Ferrous sulfate appears as a greenish or yellow-brown crystalline solid. Density 15.0 lb /gal. Melts at 64°C and loses the seven waters of hydration at 90°C. This type of anemia occurs when iron intake, iron stores, and iron loss do not adequately support the formation of erythrocytes, also known as red blood cells. Ferrous sulfate is a synthetic agent used in the treatment of iron deficiency. It is the gold standard of oral iron therapy in the UK and many other countries. Iron (2+) sulfate (anhydrous) is a compound of iron and sulfate in which the ratio of iron (2+) to sulfate ions is 1:1. Various hydrates occur naturally - most commonly the heptahydrate, which loses water to form the tetrahydrate at 57℃ and the monohydrate at 65℃. It has a role as a reducing agent. It is a metal sulfate and an iron molecular entity. It contains an iron (2+).
Application
Ferrous sulfate can be used to make iron salt, iron oxide pigment, mordant, water purifying agent, preservative, disinfectant, etc; 1. Ferrous sulfate in water treatment is used for flocculation and purification of water and removal of phosphate from urban and industrial sewage to prevent eutrophication of water body. Reducing agent a large amount of ferrous sulfate is used as reducing agent to mainly reduce chromate in cement. Medicinal ferrous sulfate is used to treat iron deficiency anemia; It is also used to add iron to food. Long term overuse may cause side effects such as abdominal pain and nausea. Medically, it can also be used as a local astringent and blood tonic, and can be used for chronic blood loss caused by hysteromyoma. Ferrous sulfate is required for the production of colorant a, iron tannate ink and other inks. The mordant used for wood dyeing also contains ferrous sulfate. Ferrous sulfate can be used to dye concrete yellow rust. Carpenters use ferrous sulfate to dye Maple with silver color. Agriculture can regulate soil pH and promote the formation of chlorophyll (also known as iron fertilizer), which can prevent the yellowing disease of flowers and trees caused by iron deficiency. It is an indispensable element of acid loving flowers and trees, especially iron trees. In agriculture, it can also be used as a pesticide to prevent wheat smut, scab of apples and pears and rot of fruit trees; It can also be used as fertilizer to remove moss and lichens from tree trunks. Analytical chemistry ferrous sulfate can be used as chromatographic reagent[1].
1.Mechanism analysis of selenium (VI) immobilization using alkaline earth metal oxides and ferrous salt. The immobilization of selenate (SeO42-) using metal oxides (CaO and MgO) and ferrous salt as the immobilization reagents were examined by the leaching test and solid-phase analysis via XRD, XAFS, TGA, and XPS. The results indicated that nearly all of SeO42ewas reduced to SeO32- in the CaO- based reaction within 7 days. Then, the generated SeO32ewas mainly sorbed onto the iron-based minerals (Fe2O3 and FeOOH) through the formation of both bidentate mononuclear edge-sharing (1E) and monodentate mononuclear corner-sharing (1V) inner-sphere surface complexes, suggested by PHREEQC simulation and EXAFS analysis[2].
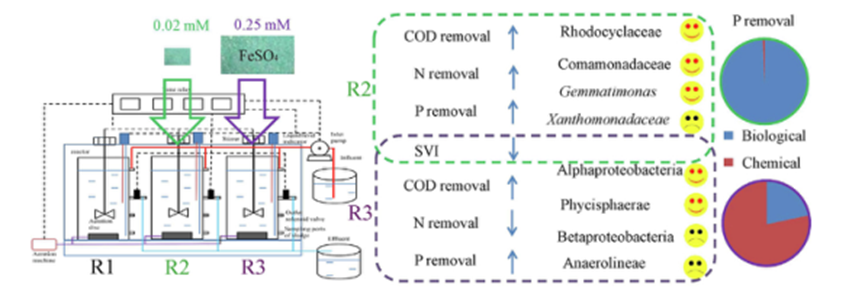
2.A novel micro-ferrous dosing strategy for enhancing biological phosphorus removal from municipal wastewater. Ferrous salts have been widely used to enhance phosphorus removal in full-scale wastewater treatment plants, with an average dosage of 0.24–0.35 mM. However, such high dosage inevitably caused serious concerns on operation, potential biological toxicity and excessive sludge production. investigated the effect of micro-dosing of ferrous salt at the level of 0.02 mM on enhanced biological phosphorus removal (EBPR) in sequencing batch reactors. Results showed that micro-dosing of ferrous salt enhanced the overall performance, with average COD, TN and TP removal of more than 4.2%, 2.0% and 5.8%, respectively. In addition, the sequencing analysis further revealed that micro-ferrous dosing could significantly improve the diversity and richness of the microbial community, whereas the regular dosing of ferrous salts (0.25 mM) negatively impacted on the EBPR performance. It was found that the abundances of phosphorus accumulating organisms (PAOs) in R2 (micro-dosing) were nearly 1.5-fold and 2-fold higher than those in R1 (control) and R3 (regular dosing). The contributions of biological and chemical pathways towards the observed phosphorus removal were also determined according to the phosphorus releasing rate. For micro-dosage and regular dosage of ferrous salts, phosphorus removal mainly relied on biological phosphorus removal and chemical phosphorus removal, respectively. It appears from this this study that the micro-ferrous dosing strategy is practically feasible and economically viable for enhanced phosphorus removal from municipal wastewater.
Figure 1 A novel micro-ferrous dosing strategy for enhancing biological phosphorus removal from municipal wastewater[3]
Synthesis
Industrial preparation method:
1. Sulfuric acid method: dissolve iron filings in the mixture of dilute sulfuric acid and mother liquor, and control the reaction temperature below 80 ℃, otherwise ferrous sulfate monohydrate will precipitate. The slightly acidic ferrous sulfate solution produced by the reaction is clarified to remove impurities, then cooled and centrifuged to obtain light green ferrous sulfate.
2. By product method of titanium dioxide production: when ilmenite is decomposed into titanium dioxide with sulfuric acid, ferrous sulfate and ferric sulfate are generated, and trivalent iron is reduced to divalent iron with iron wire. Ferrous sulfate can be obtained by freezing crystallization.
3. Dissolve 200 parts of industrial ferrous sulfate with 400 parts of distilled water at 70 ~ 80 ℃, add a small amount of silver sulfate to the hot solution and boil with steam to remove Cl -. Cool the solution with qualified Cl - content, adjust pH = 5 ~ 6, and pass hydrogen sulfide to make the plasma content of Zn2 + and Cu2 + qualified. Then filter, and the filtrate must be clear. Boil the filtrate with steam for 1h, filter it after the precipitation is complete, adjust the pH of the filtrate with chemical pure sulfuric acid to 1 ~ 2, evaporate and concentrate to 38 ~ 40 ℃, filter it while hot, adjust the pH of the filtrate with chemical pure sulfuric acid to 1, cool and crystallize, shake it dry, and bake it below 60 ℃ until it does not stick to the spoon. The finished product should be sealed and protected from light, and organic matter is strictly prohibited to mix. Mother liquor can be recycled.
4. Ferrous sulfate can be prepared by dissolving metallic iron in dilute sulfuric acid. The iron wire or scrap iron shall be treated with sodium hydroxide solution to remove the oil stain. After being washed with water, it shall be put into 15% ~ 20% sulfuric acid solution and heated to dissolve it until the insoluble residue is no longer dissolved. Filter out the solution and transfer it to the flask, add sulfuric acid and acidify it to Congo red to form an acidic reaction. After cooling, saturate with hydrogen sulfide, plug the bottle tightly and let it stand for 2 ~ 3 days, then put the flask on a water bath to heat and filter to remove carbon, carbide and sulfide precipitation. Transfer the filtrate to the fouse distillation flask, evaporate and concentrate the solution to half of the original volume under the condition of introducing oxygen free CO2. Let the solution stand in CO2 gas overnight to precipitate the crystallization of ferrous sulfate.
Safety
The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used for water or sewage treatment, as a fertilizer ingredient. Iron deficiency anemia is a large public health concern worldwide, especially in young children, infants, and women of childbearing age.
References
1.Zeng Youqun: ferrous salt treatment of potassium ferricyanide wastewater, Guangdong chemical industry, 2015, issue 11, pages 186-187.
2.Tian Q., Guo B. & Chuaicham C. et al., "Mechanism analysis of selenium (VI) immobilization using alkaline-earth metal oxides and ferrous salt," Chemosphere, Vol.248(2020), p.126123.
3.Ji B., Zhu L. & Wang S. et al., "A novel micro-ferrous dosing strategy for enhancing biological phosphorus removal from municipal wastewater," Science of The Total Environment, Vol.704(2020), p.135453.
You may like
Related articles And Qustion
See also
Lastest Price from Ferrous sulfate manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7720-78-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $3.60/kg2025-04-18
- CAS:
- 7720-78-7
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 3000tons/month