Synthesis of Biotin
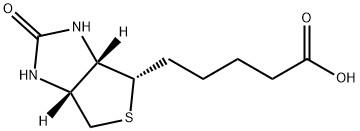
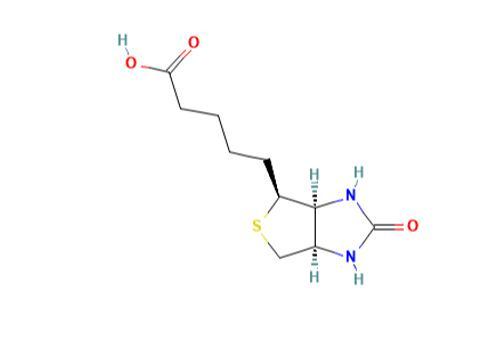
Biotin is an organic heterobicyclic compound that consists of 2-oxohexahydro-1H-thieno[3,4-d]imidazole having a valeric acid substituent attached to the tetrahydrothiophene ring. The parent of the class of biotins. Growth factor present in minute amounts in every living cell. It occurs mainly bound to proteins or polypeptides and is abundant in liver, kidney, pancreas, yeast, and milk. The biotin content of cancerous tissue is higher than that of normal tissue.
Synthesis
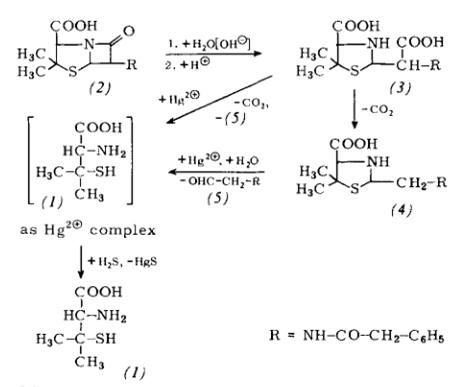
1.large-scale preparation of this vitamin employing the F. Hoffmann–La Roche lactone–thiolactone approach, developed by Goldberg and Sternbach in 1949
2.Synthetic Route to d-Biotin (1):
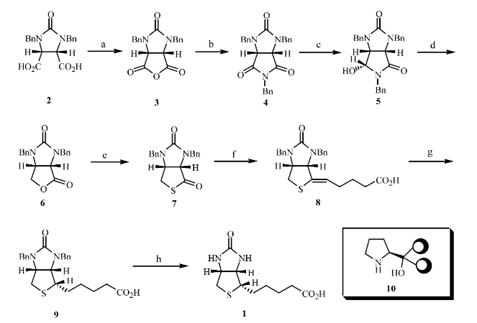
Developing a new oxazaborolidine-catalyzed reducing system that can easily be employed and that gives high enantiomeric excess for a large-scale conversion of 4 into 5 as a precursor for the formation of 6, to complete the asymmetric synthesis of 1. The known meso-cyclic-1,2-dicarboxylic anhydride 3 was pre-pared in almost quantitative yield by heating 2 in xylene with a catalytic amount of Ac2O with azeotropic removal of H2O for 13 h.
Treatment of 3 with benzylamine in toluene under reflux for 6h afforded the meso-cyclic imide 4 in a 90% yield. Next, we embarked upon the development of an efficient and convenient strategy for the large-scale asymmetric borane reduction of 4 into (3aS,6R,6aR)-hydroxylactam 5 using a chiral polymer-supported oxazaborolidine derived from polymer-supported ligand 10. Stereospecific hydrogenation of 8 was carried out under 4 atm of H2in the presence of Pd (OH)2over charcoal to give the N, N-dibenzylbiotin 9 in 95% yield. Removal of the N-benzyl group was conducted with CH3SO3H–AcOH–H2O (1.5 : 1.5 : 1) under reflux for 10h to afford d-biotin (1) in 80% yield.[1]
Application
Biotin has a role as a prosthetic group, a coenzyme, a nutraceutical, a B vitamin, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a cofactor. It is a conjugate acid of a biotinate. Ferrocene conjugates with bioactive vectors play an important role in bioorganometallic chemistry studies, mainly because these compounds demonstrate higher anticancer activity than their mother compounds.[1] The fundamental study of using of the ferrocene derivatives as an anticancer agents were performed by Prof. Jaouen and co-workers.[2] They showed that some of the ferrocenyl compounds exhibit high anticancer activity against many different types of cancer cell lines. Subsequently, many different types of ferrocenyl compounds have been synthesized and their anticancer, [1c, 1e, 3] antimalarial, [1e, 4] and antimicrobial [3b, 5] activities have been investigated.
They also exhibited high cytotoxicity toward cancer cells expressing various levels of sodium-dependent multivitamin transporter (SMVT) in the absence of biotin in the medium, whereas the presence of free biotin decreased their antiproliferative activity. This revealed that these biotin-ferrocene conjugates might be used as biologically active agents against cancer cells, although there was no clear relationship between their cytotoxicity and cellular SMVT level.biotin is a cofactor involved in carbon dioxide transfer by carboxylase enzymes in humans. Thus, it is indispensable for fatty acid synthesis and gluconeogenesis as well as metabolism of branched amino acids. It is also involved in cell signaling, epigenetic regulations of genes, and chromatin structure.[2]
Toxicological and Safety
Prolonged skin contact may cause irritation.
Handling and storage
The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational exposure or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material's impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations.
Reference
[1]Chen F. E., Jia H. Q. & Chen X. X. et al., "Synthetic studies on d-biotin, part 9. An improved asymmetric synthetic route to d-biotin via Hoffmann-Roche lactone-thiolactone approach," Chem Pharm Bull (Tokyo), Vol.53, No.7(2005), pp.743-746.
[2]
You may like
Related articles And Qustion
See also
Lastest Price from D-Biotin manufacturers

US $0.00-0.00/KG2025-11-28
- CAS:
- 58-85-5
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $1200.00-1100.00/ton2025-09-10
- CAS:
- 58-85-5
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M