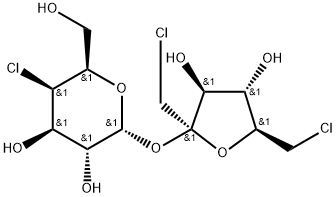
Sucralose: A Comprehensive Overview of Its Composition, Synthesis, and Applications
Sucralose, also known as sucralose, is a non-caloric, high-intensity sweetener made from sucrose. It is a white powder, odorless, non-hygroscopic, highly thermally stable, easily soluble in water, and soluble in organic solvents such as ethanol and methanol.

Figure 1 Characteristics of Sucralose
The solubility is 28.2g at room temperature of 28°C, and it has high stability under light environment, heat environment and pH value changes. The sweetness of sucralose is 600~800 times that of sucrose, and its sweetness is basically close to that of sucrose, and it will not cause tooth decay in consumers.
The sucralose aqueous solution is clear and transparent, with a pH value of 5, high stability, no chemical changes after storage for more than 1 year, and no chemical changes in crystals after storage for about 4 years, and the sweetness will not change under high temperature conditions.
Application
Sucralose can be widely used in dairy products, chewing gum, ice cream, cakes, breakfast cereals, jelly, pudding, fillings, candy, processed fruits, sauces, jams and other foods, and is also commonly used in carbonated beverages, sparkling water, tea beverages, fruit juice beverages, solid beverages, wine, beer and other beverages. At the same time, sucralose can also be added to personal health (such as mouthwash, toothpaste), medicine (cough syrup) and dietary nutritional supplements. While complying with the trend of reducing sugar in diet and reducing raw materials and operating costs, the use of sucralose can effectively reduce the area of sugarcane planting, transportation and carbon emissions, and contribute to environmental protection.
Production method
Sucralose is prepared by tritylation (shielding three primary hydroxyl groups), acetylation (shielding five secondary hydroxyl groups), detritylation, acetyl migration, chlorination, deacetylation and other reactions using sucralose as raw material. There are three methods for preparing sucralose: monoester method, enzyme-chemical method and group migration method. Since the first two methods only protect a certain hydroxyl group, the degree of substitution cannot be controlled during the chlorination process, and the resulting product is a mixture. In particular, the monoester method may cause sucrose to decompose into fructose esters. The group migration method protects multiple hydroxyl groups from the beginning, making the chlorination reaction directional, and a relatively simple product can be obtained without special separation technology, with a yield of up to 36%.
You may like
See also
Lastest Price from Sucralose manufacturers

US $0.00/kg2025-09-12
- CAS:
- 56038-13-2
- Min. Order:
- 1kg
- Purity:
- 98%-102%
- Supply Ability:
- 2000kg

US $1.00/PCS2025-04-21
- CAS:
- 56038-13-2
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10mt