Study of 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol under different condition
Introduction
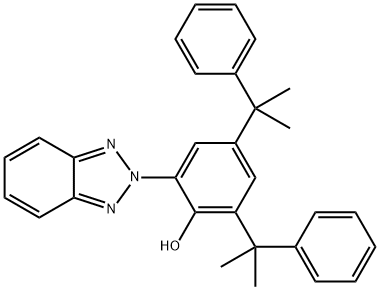
2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol is also known as UV234 (Figure 1). 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol is a benzotriazole ultraviolet absorber with a high molecular weight of 447.6. 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol is characterized by low volatility, excellent UV absorption and good compatibility with various materials. This makes it especially suitable for products with high surface area, such as films and fibers. Secondly, 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol has good heat resistance, which is more than 40℃ higher than uv327. It is especially suitable for polymers that usually need to be processed at high temperatures, such as polycarbonate, polyester, polyaldehyde resin, polyamide, polyphenylene sulfide, polyphenylene oxide, aromatic copolymers, thermoplastic polyurethane and polyurethane fibers, as well as homopolymer and copolymer of PVC and styrene. In addition, 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol has low toxicity and has been approved in many countries for polymer products in contact with food, especially for packaging food, drugs, etc.[1]

In a freshwater ecosystem and the adjacent riparian environment
Substituted diphenylamine antioxidants (SDPAs) and benzotriazole UV stabilizers (BZT-UVs) are industrial additives of emerging environmental concern. However, little is known about their environmental fate and bioaccumulation. This study investigated the concentrations of SDPAs and BZT-UVs in the water, sediment and biota samples in the freshwater ecosystem and adjacent riparian environment using Hamilton Harbour in the Great Lakes of North America as a study site. The bioaccumulation factors and trophodynamics of these contaminants were studied using field-collected samples. Eight target SDPAs and two BZT-UVs (2-(2H-benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol (UV234) and 2-(2H-benzotriazol-2-yl)-4,6-di-tert-pentylphenol (UV328)) were frequently detected in the sediment, water and biota samples. UV328 showed significantly greater concentrations in water (0.28-2.8 ng L-1) and sediment (8.3-48 ng g-1, dry weight) than other target contaminants, implying greater contamination of UV328 in Hamilton Harbour. SDPAs exhibited trophic dilution in species living in the water, whereas 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol) was biomagnified in the same samples. No clear trophodynamic trend was found for UV328 for water-respiring species. Air-breathing invertebrates had higher concentrations of both SDPAs and BZT-UVs than water-respiring invertebrates, and biomagnification was observed particularly for adult dragonflies. These results suggest that the trophodynamics of SDPAs and BZT-UVs vary depending on whether the food web is terrestrial or aquatic. Future research should investigate the occurrence and partitioning of SDPAs and BZT-UVs in the air-water interface and evaluate the toxicities of these contaminants in air-breathing species.[2]
In Drinking Water
Exposure to environmental endocrine-disrupting chemicals (EDCs) can cause extensive health issues. However, specific EDCs remain elusive. This work aimed at performing nontargeted identification of estrogen receptor α (ERα)-active compounds using an ERα protein affinity assay combined with high-resolution mass spectrometry in the source and drinking water sampled from major rivers in China. Fifty-one potential ERα-active compounds across 13 categories were identified. For the first time, diisodecyl phenyl phosphate was found to have antiestrogenic activity, and three chemicals (galaxolidone, bensulfuron methyl, and 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol) were plausible ERα ligands. Among the 51 identified compounds, 12 were detected in the aquatic environment for the first time, and the concentration of N-phenyl-2-naphthylamine, a widely used antioxidant in rubber products, was up to 1469 and 1190 ng/L in source and drinking water, respectively. This study demonstrated the widespread presence of known and unknown ERα estrogenic and antiestrogenic pollutants in the major rivers that serve as key sources of drinking water in China and the low removal efficiency of these chemicals in drinking water treatment plants.[3]
In marine wildlife of the Pearl River Estuarine
Bioaccumulation and trophic transfer in ecosystems is an important criterion for assessing environmental risks of contaminants. This study investigated bioaccumulation and biomagnification of 13 organic ultraviolet absorbents (UVAs) in marine wildlife organisms in the Pearl River Estuary, South China Sea. The UVAs could accumulate in the organisms with biota - sediment accumulation factors (BSAF) of 0.003-2.152. UV531 was the most abundant and showed the highest tendency to accumulate in the organisms with a median BSAF of 1.105. The UVAs demonstrated species - and compound-specific accumulation in the marine organism. Fishes showed significantly higher capability than the cephalopods and crustaceans in accumulation of the UVAs. Habitat did not demonstrate obvious impact on accumulation of the UVA. On the other hand, benzophenone-3, UV328, and 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol) showed significantly higher concentration in the detritus feeding fishes than carnivorous and planktivorous fishes, suggesting governing effect of dietary habits of the organisms on bioaccumulation of these UVAs. Direct uptake from growth media was a significant exposure pathway of the organisms to the UVAs. The estimated trophic magnification factors and biomagnification factors revealed that UV329, UV531, and octocrylene could potentially biomagnify in the marine food web.[4]
References
[1] Jiang YB.Synthesis of UV absorber 234[D].Dalian University of Technology,2002.
[2] Lu Z, De Silva AO, Spencer C, Tetreault GR, de Solla SR, Muir DCG. Distribution and trophodynamics of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in a freshwater ecosystem and the adjacent riparian environment. Environ Sci Process Impacts. 2024;26(6):1031-1041. Published 2024 Jun 19. doi:10.1039/d4em00193a
[3] Li Q, Wang L, Jia Y, Yang M, Zhang H, Hu J. Nontargeted Analysis Reveals a Broad Range of Bioactive Pollutants in Drinking Water by Estrogen Receptor Affinity-Mass Spectrometry. Environ Sci Technol. 2023;57(50):21327-21336. doi:10.1021/acs.est.3c05060
[4] Peng X, Fan Y, Jin J, Xiong S, Liu J, Tang C. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ Pollut. 2017;225:55-65. doi:10.1016/j.envpol.2017.03.035
You may like
Lastest Price from 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 70321-86-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200mt/year

US $0.00-0.00/KG2025-04-15
- CAS:
- 70321-86-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg