Propoxate: Pharmacology, Anesthetic Applications, and Emerging Risks
Introduction
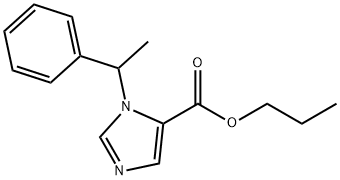
Propoxates is the proposed generic name for dl-1-(1-phenyl-ethyl)-5-(propoxy-carbonyl)-imidazole HCl. It is a member of a new series of 1-substituted imidazole-5-carboxylic acid esters. Propoxate is a short-acting anesthetic structurally related to etomidate.

Potent Anaesthetic Agent
Propoxate (R7464) is a potent, polyvalent and safe anaesthetic in cold-blooded vertebrates and is very soluble in fresh and salt water. Compared with ‘Tricaine’ (methanesulphonate of meta-amino-benzoic acid ethylester, MS222) it is about 100 times more potent, the active concentration ranging from 0.5 to 10 p.p.m. and from 50 to 1,000 p.pm. for R7464 and MS333 respectively. The degree of anaeasthesia obtained in goldfish with propoxate is closely related to the concentration used, and various and selected depths of anaesthesia can be induced with atoxic concentrations. A slight, long-lasting tranquillization, resulting in a loss of reactivity and a decreased oxygen consumption, useful for transport, can be obtained with concentrations up to 1/4 p.p.m. A somewhat deeper level of anaesthesia inducing loss of righting reflex is obtained with 1 and 4 p.p.m. At this depth routine biological investigations can be easily performed and goldfish can be remove from the water for several hours without harmful effects. The two highest concentrations shown lead rapidly to complete respiratory arrest which can be sustained for a limited period without harmful effects-1 h for 16 and 1/4 h for 64 p.p.m. The recovery time from anaesthesia depends on the concentration of propoxate and on the duration of immersion. Similar effects to those in goldfish were obtained in sunfish with the concentration of propoxate 1 and 4 p.p.m. in rainbow trout and in seafish 1/4 p.p.m. was still effective and induced stage II. In young salmon 2 p.p.m. led rapidly to loss of righting reflex. [1] [2]
Mechanism of action
Propoxate acts primarily as a positive allosteric modulator and agonist of the γ-aminobutyric acid type A (GABAA_AA) receptor, enhancing inhibitory neurotransmission in the central nervous system. By binding at sites similar to etomidate, it potentiates GABA-induced chloride currents, leading to neuronal hyperpolarization and rapid hypnosis. Its minimal effects on cardiovascular function make it advantageous over barbiturates or propofol. Potential applications include use as an intravenous anesthetic for short surgical or diagnostic procedures, induction of anesthesia before inhalational maintenance, and in veterinary anesthesia where rapid onset, smooth recovery, and hemodynamic stability are desirable.[2]
Metabolic mechanism
By using liquid chromatography-high resolution mass spectrometry (LC-HRMS), the in vivo and in vitro metabolism of propomate, an analogs of etomidate is studied in zebrafish, human liver microsomes (HLMs), human urine and hair samples. Six metamolites of propoxate were detected. The main metabolic pathways included hydroxylation, dealkylation, dehydrogenation, and glucuronidation. [3]
Usage risks
Though propoxate is used as an anaesthetic agent in cold-blooded vertebrates, it have no known therapeutic use in humans. An emerging trend of abuse propoxate has recently been observed. As an analogs of etomidate, propoxate may produces its effect by acting as a positive allosteric modulator on the γ-aminobutyric acid type A receptor and thus enhancing the effect of the inhibitory neurotransmitter γ-aminobutyric acid. It may stands out among other anesthetic agents by having a remarkably stable cardiorespiratory profile, producing no cardiovascular or respiratory depression. However, propoxate may suppresses the adrenocortical axis by the inhibition of the enzyme 11β-hydroxylase. This makes the drug unsuitable for administration by a prolonged infusion. It also makes the drug unsuitable for administration to critically ill patients. Propoxate may has relatively large volumes of distributions and is rapidly metabolized by hepatic esterases into an inactive carboxylic acid through hydrolyzation. Misuse of the compounds could be a harbinger of an increasing prevalence of acquired 11β-hydroxylase deficiency. The compounds are novel psychoactive substances. Medical practitioners should remain vigilant in detecting misuse of these compounds. The disruptions in steroidogenesis are sinister adverse effects that require increased awareness from the medical community. [3] [4]
References
[1] Thienpont, D., & Niemegeers, C. J. E. (1965). Propoxate (R 7464): a new potent anaesthetic agent in cold-blooded vertebrates. Nature, 205(4975), 1018-1019.
[2] Valk, B. I., & Struys, M. M. R. F. (2021). Etomidate and its Analogs: A Review of Pharmacokinetics and Pharmacodynamics. Clinical pharmacokinetics, 60(10), 1253–1269. https://doi.org/10.1007/s40262-021-01038-6
[3] Tang, Y., Xu, L., Zhao, J., Qian, X., Qiang, H., Xiang, P., & Yan, H. (2025). Metabolic Profile of Etomidate and Its Three Analogs in Zebrafish, Human Liver Microsomes, Human Urine and Hair Samples Using UHPLC‐Q Exactive Orbitrap‐HRMS. Drug Testing and Analysis.
[4] Cheung, Y. T., Lau, C. Y., Tseung, J. S. B., Yu, K. Y. C., Cheung, H. N., Shek, C. C., ... & Chong, Y. K. (2025). Acquired 11β-Hydroxylase deficiency in etomidate and (Iso) propoxate abusers: A nascent endocrine condition. Steroids, 109639.