Some reactions involving 2-hydroxybenzyl alcohols in synthesis
Introduction
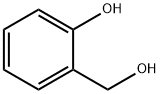
2-Hydroxybenzyl alcohol (Figure 1) is an important fine chemical product, which is widely used in the synthesis of pesticide, medicine, spice, cosmetic and so on. 2-Hydroxybenzyl alcohol is a kind of Ortho-quinone methides (o-QMs) precursors with unique advantages. Hence, the catalytic asymmetric [4+n] cycloadditions, tandem cyclizations and nucleophilic additions of o-hydroxybenzyl alcohols have developed very rapidly in recent years, which have become efficientstrategies for the synthesis of chiral oxygen-containing heterocycles and arylmethane derivatives. The reactions involving 2-hydroxybenzyl alcohols are summarized, which will open a new window for the design of new type of 2-hydroxybenzyl alcohols and their involved catalytic asymmetric reactions.[1]

Catalytic Acceptorless Dehydrogenation of Amino Alcohols and 2-Hydroxybenzyl Alcohols
A base-free and acceptorless Ru-catalyzed dehydrogenative approach has been developed for the synthesis of N-heterocycles by using 1,3-dicarbonyls and amino alcohols through a domino sequential enamine formation and intramolecular oxidative cyclization strategy. This unified approach is also applicable for the synthesis of O-heterocycles involving 2-hydroxybenzyl alcohol as a coupling reactant via consecutive C-alkylation and intramolecular cyclization steps. The present protocol is general for the synthesis of varieties of biologically important scaffolds, such as tetrahydro-4H-indol-4-one, 3,4-dihydroacridin-1(2H)-one, and tetrahydro-1H-xanthen-1-ones derivatives using a single catalytic system, viz. RuH2CO(PPh3)3. Environmentally benign H2O and H2 are the only byproducts in this domino process. Moreover, RuH2CO(PPh3)3-catalyzed C3-alkylation of tetrahydro-4H-indol-4-one using alcohol as a alkylating partner is also described in this report. For the first time, a solvent-free gram-scale reaction for the acceptorless dehydrogenative annulation has been demonstrated. A plausible mechanism for the Ru-catalyzed base-free and acceptorless dehydrogenative annulation of amino alcohols or 2-hydroxybenzyl alcohols has been provided with several experimental investigations and spectroscopic evidence.[2]
Carbonylative intramolecular synthesis
In this procedure, researchers believe the in situ formed 2-hydroxybenzyl alcohols are the key intermediate for the transformation. In addition, 2-hydroxybenzyl alcohols were produced from phenols and aldehydes using CF3CO2H as a catalyst. Theoretically, the acid additive can be successfully avoided if we directly use 2-hydroxybenzyl alcohols as the starting material. With this idea in mind and also as their continuous efforts on CO source development and heterocycles synthesis, they describe herein a palladium-catalyzed intramolecular carbonylative reaction for benzofuranone synthesis using 2-hydroxybenzyl alcohols as the starting material and formic acid as the CO source. Moderate to good yields of the desired benzofuranones were obtained.A palladium-catalyzed carbonylative intramolecular synthesis ofbenzofuran-2(3H)-ones from 2-hydroxybenzyl alcohols has been developed. In this procedure, formic acid was utilized as the CO source, and various benzofuran-2(3H)-one derivatives were obtained in moderate to good yields.[3]
Rhodium Catalyzed Synthesis of Benzopyrans
o-Quinone methides (o-QMs) are another class of short-lived and highly reactive intermediates frequently encountered in synthesis, as well as in biological applications.In synthesis, o-QMs are readily generated from 2-hydroxybenzyl alcohol derivatives and enable straightforward synthesis of various substituted (hetero) aromatics. For instance, [4+2]-annulation of o-QMs with electron-rich alkenes affords the benzopyrans, a subunit widely present in therapeutically important molecule and natural products. However, the complete potentials of o-QMs in organic synthesis are yet to be explored. An efficient and novel rhodium-catalyzed transannulation of N-sulfonyl-1,2,3-triazoles with in situ generated oquinone methides (o-QMs) from 2-hydroxybenzyl alcohols has been achieved for the synthesis of substituted benzopyrans in good yields. The developed reaction involves nucleophilic attack of o-QM to α-imino rhodium carbenoid to generate a carbonylylide followed by 6π-electrocyclization and isomerization. Furthermore, the utility of the methodology was demonstrated in the one-pot synthesis and construction of polyheteroaromatics.[4]
Effects of salicylate derivatives on SLC26A4
Pendrin is a transmembrane protein encoded by the SLC26A4 gene that functions in maintaining ion concentrations in the endolymph of the inner ear, most likely by acting as a chloride/bicarbonate transporter. Variants in the SLC26A4 gene are responsible for sensorineural hearing loss. Although pendrin localizes to the plasma membrane, we previously identified that 8 missense allele products of SLC26A4 were retained in the intracellular region and lost their anion exchange function. Researchers also found that 10 mM salicylate induced the translocation of 4 out of 8 allele products from the intracellular region to the plasma membrane and restored their anion exchanger activity. However, since 10 mM salicylate exhibits cytotoxicity, the use of chemical compounds with less cell toxicity is needed. In the present study, therefore, salicylate derivatives were used as the chemical compounds and their effects on the p.H723R allele products of SLC26A4 were investigated. HEK293 cells were transfected with the cDNA of p.H723R. Cell proliferation, viability and toxicity assays were performed to investigate the response and health of cells in culture after treatment with four types of salicylate derivatives, i.e., 2-hydroxybenzyl alcohol, 2,3-dihydroxybenzoic acid, 2'-hydroxyacetophenone and methyl salicylate. The effects of these salicylate derivatives on the localization of the p.H723R were investigated by immunofluorescence microscopy. The application of 10 mM salicylate showed an increase in cell toxicity and decrease in cell viability, leading to a significant decrease in cell proliferation. In contrast, the application of 1 mM salicylate derivatives did not show any significant increase in cell toxicity and decrease in cell viability, corresponding to a logarithmic increase in cell concentration with an increase in culture time. Immunofluorescence experiments showed that the p.H723R retained in the endoplasmic reticulum (ER). Among the salicylate derivatives applied, 2-hydroxybenzyl alcohol induced the translocation of p.H723R from the ER to the plasma membrane 3 h after its application. The results obtained showed that 2-hydroxybenzyl alcohol restored the localization of the p.H723R allele products of SLC26A4 from the ER to the plasma membrane at a concentration of 1 mM by 3 h after its administration with less cytotoxicity than 10 mM salicylate.[5]
References
[1] Wang HQ,et al.,Advances in Catalytic Asymmetric Reactions Involving o-Hydroxybenzyl Alcohols[J].Chinese Journal of Organic Chemistry,2023,43(03):974-999.
[2] Pandey AM, Digrawal NK, Mohanta N, et al. Catalytic Acceptorless Dehydrogenation of Amino Alcohols and 2-Hydroxybenzyl Alcohols for Annulation Reaction under Neutral Conditions. J Org Chem. 2021;86(13):8805-8828. doi:10.1021/acs.joc.1c00714
[3] Li HP, Ai HJ, Qi X, Peng JB, Wu XF. Palladium-catalyzed carbonylative synthesis of benzofuran-2(3H)-ones from 2-hydroxybenzyl alcohols using formic acid as the CO source. Org Biomol Chem. 2017;15(6):1343-1345. doi:10.1039/c6ob02782b
[4] Yadagiri D, Chaitanya M, Reddy ACS, Anbarasan P. Rhodium Catalyzed Synthesis of Benzopyrans via Transannulation of N-Sulfonyl-1,2,3-triazoles with 2-Hydroxybenzyl Alcohols. Org Lett. 2018;20(13):3762-3765. doi:10.1021/acs.orglett.8b01338
[5] Murakoshi M, Koike Y, Koyama S, et al. Effects of salicylate derivatives on localization of p.H723R allele product of SLC26A4. Auris Nasus Larynx. 2022;49(6):928-937. doi:10.1016/j.anl.2022.03.009
You may like
See also
Lastest Price from 2-Hydroxybenzyl alcohol manufacturers
US $0.00-0.00/KG2024-08-07
- CAS:
- 90-01-7
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000KG
US $0.00-0.00/kg2023-06-19
- CAS:
- 90-01-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- kgs