Application researches of sodium trifluoromethanesulfinate
Introduction
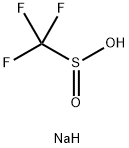
Sodium trifluoromethanesulfinate (CF3SO2Na, Langlois reagent;Figure 1) is a cheap, white solid and readily available industrial product. Sodium trifluoromethanesulfinate is an economical and environmentally friendly, chemically stable trifluoromethylation reagent, which is widely used in free-radical trifluoromethylation reactions. Several studies have shown that the trifluoromethylations of heterocycles, aryl boronic acids, α,β-unsaturated carboxylic acids, and unsaturated organotrifluoroborates could be achieved by CF3SO2Na.

Transition-Metal-Free Trifluoromethylation
A metal-free and cost-effective synthetic protocol for the trifluoromethylation of N,N-disubstituted hydrazones with sodium trifluoromethanesulfinate (CF3SO2Na) to afford the corresponding functionalized trifluoromethyl ketone hydrazones has been established. It is proposed that a radical/SET mechanism proceeding via a trifluoroalkyl radical may be involved in the reaction. Applications of the methodology in industry will be found and the development of new methods for trifluoromethylation with Langlois’s reagent will be continued.The procedure is highlighted by its operational simplicity, excellent functional group tolerance, and mild reaction conditions. This simple reaction is believed to occur by a CF3-radical-transfermechanism and provides a convenient and practical approach to useful trifluoromethylated building blocks with a wide variety of functional groups.[1]
Sodium trifluoromethanesulfinate-mediated photocatalytic strategy
1.Dibenzo-[b,f][1,4]oxazepine derivatives Synthesis
Dibenzo[b,f][1,4]oxazepine derivatives play important roles in synthetic organic chemistry and pharmaceutical science. However, the synthetic approaches to diverse dibenzo-[b,f][1,4]oxazepine derivatives are very limited. Here, sodium trifluoromethanesulfinate was applied as the precursor of the organic photocatalyst, and the selective C−H acylation of dibenzo[b,f][1,4]oxazepines with 4-acyl-1,4-dihydropyridines was performed well in air under light irradiation at room temperature,in which sodium trifluoromethanesulfinate transformed into pentacoordinate sulfide under the present conditions and the in situ-formed pentacoordinate sulfide acted as an efficient photocatalyst. The protocol was effectively extended to the selective C−H acylation of 11H-dibenzo[b,e]azepines. The present method shows some advantages, including a cheap photocatalytic system,mild conditions, a wide substrate scope, and moderate to good yields.[2]
2.Aerobic oxidation of alcohols
In addition,a sodium trifluoromethanesulfinate-mediated photocatalytic strategy for the aerobic oxidation of alcohols has been developed for the first time, and the photoredox aerobic oxidation of secondary and primary alcohols provided the corresponding ketones and carboxylic acids,respectively, in high to excellent yields.[3]
3.Aerobic oxidative esterification of aromatic aldehydes and alcohol
A sodium trifluoromethanesulfinate-mediated photocatalytic strategy for the aerobic oxidative esterification of aromatic aldehydes and alcohols has been developed, in which the in situ formed pentacoordinate sulfide derived from readily available and inexpensive sodium trifluoromethanesulfinate and oxygen acts as the photocatalyst, and the corresponding aromatic esters were provided in moderate to good yields. The present method is an economical and environmentally friendly protocol.[4]
4.Trifluoromethyl-containing oxazolines Synthesis
A transition metal-free method for the trifluoromethylation of N-allylamides has been developed, and the corresponding trifluoromethyl-containing oxazolines were prepared in moderate to good yields. The protocol uses readily available substituted N-allylamides as the starting materials, inexpensive and easily stored sodium trifluoromethanesulfinate as the trifluoromethyl source, iodobenzene diacetate as the oxidant, and the procedure involves sequential intermolecular trifluoromethylation of alkenes with sodium trifluoromethanesulfinate and intramolecular cyclization. This is the first example to prepare CF3-containing oxazolines. Therefore, the present method should afford an efficient and practical strategy for synthesis of other CF3-containing cyclic compounds.[5]
5.Aryl Triflones Synthesis
The direct synthesis of aryl triflones, that is, trifluoromethanesulfonyl arenes, was achieved through the trifluoromethanesulfonylation of benzynes. The trifluoromethanesulfonyl group, one of the fluorinated functional groups, is a highly electron-negative and mild lipophilic substituent. Aryl triflones have high potential in the synthesis of bioactive compounds and specialty materials. The treatment of 2-(trimethylsilyl)aryl trifluoromethanesulfonates with cesium fluoride in the presence of 15-crown-5 generated benzynes, which reacted with sodium trifluoromethanesulfinate followed by protonation with tBuOH under heating conditions, provided aryl triflones in moderated to good yields. Both symmetrical and unsymmetrical triflones were nicely accessed under the same reaction conditions. Interestingly, the trifluoromethanesulfonylation of unsymmetrical benzyne precursors proceeded smoothly to furnish corresponding aryl triflones in good yields with good to high regioselectivities. The balance of polarization of electric charge as well as steric hindrance of the benzyne intermediates are central factors to control the outcome of regioselectivity.[6]
References
1.Tan Z, Zhang S, Zhang Y, Li Y, Ni M, Feng B. Transition-Metal-Free Trifluoromethylation of Aldehyde Derivatives with Sodium Trifluoromethanesulfinate. J Org Chem. 2017;82(18):9384-9399. doi:10.1021/acs.joc.7b01359
2.Liu Y, Zhang Y, Xiao W, et al. Sodium Trifluoromethanesulfinate-Promoted Photocatalytic C-H Acylation of N-Heterocycles with 4-Acyl-1,4-dihydropyridines in Air. Org Lett. 2025;27(29):7914-7919. doi:10.1021/acs.orglett.5c02237
3.Zhu X, Liu C, Liu Y, Yang H, Fu H. A sodium trifluoromethanesulfinate-mediated photocatalytic strategy for aerobic oxidation of alcohols. Chem Commun (Camb). 2020;56(82):12443-12446. doi:10.1039/d0cc05799a
4.Liu Y, Zhu X, Zhang Y, Yi Z, Yang X, Fu H. Sodium trifluoromethanesulfinate-mediated photocatalytic aerobic oxidative esterification of aromatic aldehydes and alcohols. Org Biomol Chem. 2024;23(1):183-187. Published 2024 Dec 18. doi:10.1039/d4ob01476f
5.Yu J, Yang H, Fu H. Transition metal-Free trifluoromethylation of N-allylamides with sodium trifluoromethanesulfinate: Synthesis of trifluoromethyl-containing oxazolines. Adv. Synth. Catal. 2014, 356, 3669−3675.
6.Sumii Y, Sugita Y, Tokunaga E, Shibata N. Synthesis of Aryl Triflones through the Trifluoromethanesulfonylation of Benzynes. ChemistryOpen. 2018;7(2):204-211. Published 2018 Feb 27. doi:10.1002/open.201700204
You may like
See also
Lastest Price from Sodium trifluoromethanesulfinate manufacturers
US $0.00/KG2025-11-21
- CAS:
- 2926-29-6
- Min. Order:
- 2000KG
- Purity:
- 99.9%
- Supply Ability:
- 20tons
US $10.00/KG2025-04-21
- CAS:
- 2926-29-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt