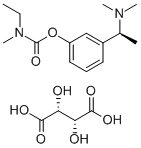
Rivastigmine Tartrate: From Mechanism to Long-Term Alzheimer's Care
Rivastigmine tartrate is used for the management of dementia of the Alzheimer's type (Alzheimer's disease). The currently available oral dosage forms (capsules and oral solution) are FDA-labeled for use in patients with mild to moderate disease, and the transdermal system is FDA-labeled for use in patients with mild, moderate, or severe disease. Patients receiving transdermal Rivastigmine tartrate therapy or their caregivers should be carefully instructed in the use of the transdermal system. To expose the adhesive surface of the system, the protective liner should be peeled and discarded prior to administration. The transdermal system is applied topically to a dry, hairless area of intact skin, preferably the back, by firmly pressing the system with the adhesive side touching the skin; placement on the back is recommended to reduce the risk that the system could be removed by the patient.

Rivastigmine for Alzheimer's disease
Alzheimer's disease (AD), alone or in combination with other brain conditions, is the commonest cause of dementia affecting older people. It is associated with the loss of cholinergic neurons in parts of the brain subserving aspects of memory. As the disease progresses, people lose the ability to remember, communicate, think clearly and perform their usual daily activities. Their behaviour or personality may also change. In severe AD, people lose the ability to care for themselves and require full time care. Rivastigmine tartrate is a 'pseudo‐irreversible' inhibitor of acetyl and butyrylcholinesterases with a phenylcarbamate structure, the metabolism of which is almost totally independent of the hepatic cytochrome P450 system. After binding to cholinesterase, the carbamate portion of rivastigmine is slowly hydrolysed, cleaved, conjugated to a sulphate and excreted. Rivastigmine tartrate has an oral bioavailability of 0.355 and low (40%) binding to plasma proteins. Its elimination half‐life is around two hours. Its disposition is essentially unaltered in patients with renal or hepatic impairment (Jann 2000) and the risk of interactions with other drugs is low. This is of particular relevance for elderly patients with AD, some of whom may also need medications for other conditions.[1]
The two cognitive tests used, the MMSE and ADAS‐Cog, assess similar domains and a high correlation between the results would be expected. The results from 5 studies show that 6 to 12 mg daily of oral Rivastigmine tartrate improved the cognitive function of patients with mild to moderate probable Alzheimer's disease treated over a period of 26 weeks, by 0.8 points on the MMSE (range 0 to 30) and by 2.0 points on the ADAS‐Cog (range 0 to 70), when compared with placebo. Four studies assessed the effect of 6 to 12 mg daily oral rivastigmine on activities of daily living as reported by a carer using the PDS rating scale (range 0 to 100). Rivastigmine tartrate showed a benefit of 2.2 points compared with placebo, but the difference between placebo and 1 to 4 mg daily Rivastigmine tartrate was not significant. The 10 cm2 (9.5 mg/day) patch showed a benefit of 2.2 points on the ADCS‐ADL scale (range 0 to 54) when compared with placebo. The US Food and Drug Administration (FDA) requires an independent clinician to assess global clinical state after interviewing the patient and the carer at baseline and the endpoint. When the results of global impression measures were dichotomized to compare the number of patients who improved with the numbers who showed no change or whose condition had deteriorated.
Rivastigmine tartrate: an open-label, observational study of safety and effectiveness
Rivastigmine tartrate's approval by the FDA in 2000 was supported by several pivotal trials, including a randomized US trial (ENA 713 B352). In this pivotal trial, 699 patients with mild to moderately severe AD were randomized to high dose rivastigmine (6–12 mg/day), low dose (1–4 mg/day) or placebo with a 7 week fixed dose-titration phase followed by a flexible dosing phase during weeks 8–26. Results of the 26-week open-label extension of this study found that at 52 weeks, patients originally treated with 6–12 mg/day Rivastigmine tartrate had significantly better cognitive function than patients originally treated with placebo. In this paper the authors present descriptive findings for a cohort of 37 patients who participated in the long-term open-label extension of the ENA713B352 rivastigmine trial. Findings presented in this article will add to the current limited dataset for long-term efficacy and outcomes with cholinesterase inhibitor therapy for persons with probable AD. This report describes our experience in following the cohort of patients at our center with AD treated with the ChE inhibitor Rivastigmine tartrate (a medication that inhibits both butyl- and acetylcholinesterase) as part of the ENA 713 B352 pivotal trial for a period up to 5 years.[2]
In summary, long-term therapy with the ChE inhibitor rivastigmine was well tolerated with no dropouts due to adverse effects past the first 9 months of the open-label extension. In contrast to the generally reported community experience of relatively brief duration of therapy with ChE inhibitors in AD, 25% of patients in this study were still taking Rivastigmine tartrate by the end of 5 years. Of interest is the multifactorial nature of reasons for treatment discontinuation. Only 1 of these patients withdrew from the study because of perceived treatment failure over the 5-year period. Disease progression accounted for the withdrawal of 4 patients. These results are informative both for the duration of treatment, and because the majority of the patients who continued treatment during this 5 year period were from the group originally titrated up to a high dose during the initial double-blind phase. Given that the sample size prohibits significance testing, these data suggest that Rivastigmine tartrate therapy may be sustained over long periods of time in a significant percentage of patients with AD, and that high-dose therapy, particularly when begun early, may have an advantage in delaying the progression of the illness.
References
[1]Birks JS, Chong LY, Grimley Evans J. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev. 2015 Sep 22;9(9):CD001191. doi: 10.1002/14651858.CD001191.pub4. PMID: 26393402; PMCID: PMC7050299.
[2]Onor ML, Trevisiol M, Aguglia E. Rivastigmine in the treatment of Alzheimer's disease: an update. Clin Interv Aging. 2007;2(1):17-32. doi: 10.2147/ciia.2007.2.1.17. PMID: 18044073; PMCID: PMC2684084.
Related articles And Qustion
Lastest Price from Rivastigmine tartrate manufacturers
US $0.00-0.00/g2025-04-18
- CAS:
- 129101-54-8
- Min. Order:
- 100g
- Purity:
- 99%
- Supply Ability:
- 5kg

US $0.00/kg2025-03-07
- CAS:
- 129101-54-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons