D-Tryptophan Methyl Ester Hydrochloride: An Immunomodulator Regulating Tryptophan Metabolism
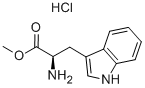
D-Tryptophan methyl ester hydrochloride is a significant chiral amino acid derivative, representing the methyl ester hydrochloride form of tryptophan. It holds irreplaceable application value within the fields of pharmaceuticals, biochemistry, and organic synthesis. From a molecular structural perspective, it is derived from D-configuration tryptophan. The carboxyl group (-COOH) in the tryptophan molecule undergoes esterification with methanol to form a methyl ester group (-COOCH₃). Simultaneously, acidification with hydrochloric acid produces the hydrochloride salt. This structural modification not only enhances its chemical stability but also improves water solubility. Compared to free D- -tryptophan methyl ester, the hydrochloride salt form exhibits superior solubility in polar solvents such as water, methanol, and ethanol, facilitating subsequent experimental procedures and pharmaceutical formulation development. Furthermore, the retained indole ring structure confers characteristic ultraviolet absorption (typically around 280 nm), enabling quantitative detection via UV spectrophotometry. The D-chiral configuration determines its unique biological activity and stereoselectivity, distinguishing it from naturally occurring L-tryptophan derivatives. Regarding physicochemical properties, D-tryptophan methyl ester hydrochloride typically presents as a white to off-white crystalline powder with a melting point of approximately 180–185°C.

The Immunomodulator D-Tryptophan methyl ester hydrochloride
D-Tryptophan methyl ester hydrochloride (1-MT) is a TRP analog described first in 1991 as a potential competitive inhibitor of the enzyme indoleamine 2,3-dioxygenase (IDO1). IDO1 is one rate-limiting enzyme of the kynurenine pathway (KP), which plays a crucial role in the regulation of the immune response, notably as a counter-regulatory mechanism in the context of inflammation. In cell free assays, it has been shown that D-Tryptophan methyl ester hydrochloride binds to the ferrous IDO complex but cannot be catalytically converted to kynurenine due to the additional methyl group. 1-MT is known for its low toxicity and great pharmacokinetic properties such as good intestinal absorption, low clearance, and low binding to plasmatic proteins. It has been reported that the application of 1-MT in pigs resulted in increased plasma levels of KYNA of around 5 μM which is sufficient to activate AhR and GPR35 as KYNA has a great affinity to the latter even at low micromolar range. In addition, these observations are further supported ex vivo in murine immune cells. Treatment of murine splenocytes with 5 μM KYNA exerted a slight proliferative effect concurrent with increased secretion of IL-1β and IL-6, suggesting that D-Tryptophan methyl ester hydrochloride mediates its biological and yet significant effects via AhR and/or GPR35.[1]
In this study, effects of 1-MT on TRP metabolism were investigated in mice and humans to provide additional insights into potential modes of action of D-Tryptophan methyl ester hydrochloride as previously described in mice and pigs. The hydrochloride salt readily dissociates into hydrogen ions and chloride ions in aqueous solution, maintaining a weakly acidic pH. The results of experiment 1 show that 1-MT increased the concentrations of TRP in supernatants of cultured murine splenocytes, human blood cell culture, and murine plasma. The results from the present study indicate that the administration of D-Tryptophan methyl ester hydrochloride induces a shift of KP toward the branch of KYNA. This effect was shown independently in two mice strains as well as in human blood. Furthermore, the results from IDO knockout mice, as well as the decreased levels of KYN in vivo, indicate that the 1-MT-induced increase of KYNA seems not to be dependent on IDO1 activity. The increase of KYNA may be one potential mode of action by 1-MT and should be considered for preclinical studies and therapeutic applications in humans. Furthermore, the application of D-Tryptophan methyl ester hydrochloride might have therapeutic implications as it may provide a method to increase KYNA while preserving IDO1 activity. Due to its immunoregulatory role, the inhibition of IDO1 may not be appropriate for different therapeutic approaches.
References
[1]Wirthgen E, Leonard AK, Scharf C, Domanska G. The Immunomodulator 1-Methyltryptophan Drives Tryptophan Catabolism Toward the Kynurenic Acid Branch. Front Immunol. 2020 Feb 28;11:313. doi: 10.3389/fimmu.2020.00313. PMID: 32180772; PMCID: PMC7059861.
See also
Lastest Price from D-Tryptophan methyl ester hydrochloride manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 14907-27-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 500kgs

US $100.00-75.00/kg2025-04-21
- CAS:
- 14907-27-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000