Research on the Mechanism and Synthesis of Methoxyamine Hydrochloride
Introduction
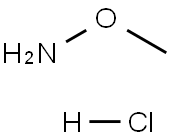
Methoxyamine (O-Methylhydroxylamine; Figure1) hydrochloride is an orally active and potent base excision repair (BER) inhibitor. Methoxyamine hydrochloride binds to 3-hydroxyl groups that are left behind by 3-methylpurine-DNA glycosylase (MPG) following excision of the damaged base and thus inhibits BER activity. Methoxyamine hydrochloride binds directly to the apyrimidinic (AP) sites. Methoxyamine hydrochloride synergistically enhances the therapeutic efficacy of DNA-damaging agents[1][2].As a chemical intermediate, methoxyamine hydrochloride is mainly used for introducing methoxyamine group in organic synthesis, which is widely applied to cefuroxime and kresoxim-methyl production.

Basic research on mechanism
Effect of O-methylhydroxylamine (Methoxyamine hydrochloride) on extracellular phage cd
Study was made of lethal and mutagenic effect of 1 M and 0,5 M O-methylhydroxylamine (OMHA) on extracellular phage Sd. The correlation between chemical changes of the genome and the degree of phage inactivation under the action of OMHA has been established within the range of studied pH (4,5-7,0) of the reaction medium. OMHA in activates the phage at the highest rate at pH 5,0, which agrees with chemical data indicating that the total rate of OMHA modification of cytidine units is maximal at this pH. Inactivation curves of OMHA-treated phage are single-hit at pH investigated, but have a small initial shoulder; at pH 5,0 and 4,5 inactivation curves consist of two exponents, the second exponent having the smallest slope, that is the phage is characterized by an increased resistance to OMHA at this section. The increased phage resistance can be explained by transforming the original product IV (cross-linked with protein) into the product II (N4-methoxy-6-methoxyamine-5,6-dihydrocytidine) which can be repaired in contrast to IV. OMHA has a high mutagenic effect on phage Sd. Under optimal conditions (at pH 4,5) the mutagen induces plaque mutants (up to 6%) among survived phages. The data obtained correlate with the fact that with decreasing pH (from 5,0 to 4,5) the ratio of the "mutagen" unit - N4-methoxycytidine (product III) to the "inactivating" one (product II) increases. The curves of mutation induction under the action of OMHA have a characteristic form with the initial linear section and the maximum or the plateau similar to mutation curves to be observed under the action of radiation and chemical agents.[3]
Induction of direct mutations of intracellular phage cd exposed to methoxyamine hydrochloride
Intracellular development of DNA-containing cd phage in the presence of methoxyamine hydrochloride (in vivo mutagenesis) results in 50-fold increase of mutants in the phage progeny. The main effect is due to the mutagen presence during replication of phage DNA (within 10-20 min after the infection). The presence of the mutagen both before and after DNA replication does not produce any considerable mutagenic effect. Comparison of the data obtained with kinetic reaction of methoxyamine hydrochloride with nucleic acid components is due to enzymatic formation of modified precursors, N4-metoxycytidine and/or N6-metoxyadenosine derivatives, which have dual functional specificity, and to their incorporation into genome under DNA replication. The presence of methoxyamine hydrochloride increases not only the number of mixed clones with a high content of mutants, but also the number of pure mutant clones. Recombinogenic activity of methoxyamine hydrochloride is considered to be a possible cause of this effect.[4]
Inactivating effect of methoxyamine hydrochloride on phage lambda and its mutants
The kinetics of inactivation of lambda+, lambda C160, lambda Nsus7, lambda Gsus9, lambda Tsus6 phages under the effect of methoxyamine hydrochloride was studied. Inactivation curves of all the phages under study were found to be of a complex character and early in the reaction (up to 4 hours) to deviate from exponential dependence of the inactivation rate upon the time of incubation with the mutagen. All the phages under study showed differences in the inactivation rates early in the reaction. Constants of the inactivation rate in the linear part of the curve vary from 30 hours-1 for lambda+ to 0.14 hours-1 for lambda C160. [5]
Mutagenic effect of methoxyamine hydrochloride on the prophage and extracellular phage lambda
Induction of c-mutations in extracellular bacteriophage and prophage lambda cI857 ind-treated with 1 M methoxyamine hydrochloride at 32 degrees and pH 5.6 has been studied. The frequency of c-mutations increases proportionally to the time of treatment of extracellular phage and does not depend on cellular recA+ or polA+ functions and on induction of SOS-repair system caused by UV-irradiation of host cells. Prophage is inactivated and mutagenized approximately 10-fold faster than extracellular phage immediately after treatment of lysogenic cells during prophage induction. Thus, prophage survival does not depend on repair functions of the host cells, and the frequency of c-mutations in recA and, especially, in polA lysogens is significantly lower, than in the wild-type cells. Delayed thermoinduction (90 min) of prophage causes significant enhancement of survival and decreases the frequency of c-mutations in all strains studied. Preliminary treatment of non-lysogens with methoxyamine hydrochloride does not increase the frequency of c-mutations in undamaged phage or in phage treated with methoxyamine hydrochloride in vitro.[6]
An optimized synthesis method of methoxyamine hydrochloride
In Hu's thesis, the methylation and hydrolysis of methoxyamine hydrochloride were studied respectively. The selection of methylation reagent, the ratio of temperature and acid-binding agent were studied in the methylation process. In terms of hydrolysis, the temperature control, the acid ratio was of crucial importance on product yield and quality. According to the research, we obtained the optimal reaction conditions: The methylation reaction was carried out using water and PEG 500 (poly(ethylene glycol) 500) as solvent and dimethyl sulfate as the methylation reagent in the molar ratio of butanone oxime and dimethyl sulfate was 1.00:1.15. The reactive temperature was controlled at 15-20℃, and the reaction was added dropwise for 3h. After the reaction was completed, methanol and butanone oxime were distilled off. Hydrolysis reaction is a distillation hydrolysis reaction, mixed with 20% diluted hydrochloric acid (the molar ratio of hydrochloric acid and butanone oxime methyl ether was 1.15:1.00), adjusting the top reflux ratio column overhead temperature control <80℃to recover butanone. The hydrochloride solution was continuously taken out from bottom and concentrated to obtain the crude product. Finally, the trial total yield is up to 82%.
According to the experimental result, a scheme of the production of 2000 t/a methoxyamine hydrochloride coupled with industrialization was designed, and the detailed industrialization design was carried out for the continuous hydrolysis.[7]
References
[1] Sameer Agnihotri, et al. ATM regulates 3-methylpurine-DNA glycosylase and promotes therapeutic resistance to alkylating agents. Cancer Discov. 2014, 4(10):1198-213.
[2] Samideh Khoei, et al. Effects of resveratrol and methoxyamine on the radiosensitivity of iododeoxyuridine in U87MG glioblastoma cell line. Exp Biol Med (Maywood). 2016, 241(11):1229-36.
[3] Preobrazhenskaia E S , Budovski E T , Kriviski A S. Effect of O-methylhydroxylamine on extracellular phage cd. Genetika, 1976, 12(8):116-123.
[4] Preobrazhenskaia E S , Klebanova L M , Budovski E I ,et al. Induction of direct mutations of intracellular phage cd exposed to O-methylhydroxylamine Genetika, 1978, 14(5):877-889.
[5] Tkhruni F N , Kiseleva N P , Komarova E A ,et al. Inactivating effect of o-methylhydroxylamine on phage lambda and its mutants. Voprosy Virusologii, 1975, (3):367.
[6] Kalinin VL, Kuznetsova LV, Perumov DA. Mutagennoe deĭstvie o-metilgidroksilamina na profag i vnekletochnyĭ fag lambda. Mol Gen Mikrobiol Virusol. 1985, (5):23-28.
[7] Hu Q M. Study on the improvement of synthesis process of methoxyamine hydrochloride[D]. Zhejiang University of Technology,2019.
Related articles And Qustion
See also
Lastest Price from Methoxyamine Hydrochloride manufacturers

US $0.00-0.00/ton2025-06-10
- CAS:
- 593-56-6
- Min. Order:
- 1ton
- Purity:
- 98%
- Supply Ability:
- 100

US $1.00/KG2025-04-21
- CAS:
- 593-56-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt