Regorafenib for the Treatment of Sarcoma
General Description
Regorafenib, a multikinase inhibitor, has shown potential in the treatment of non-GIST sarcomas. It targets multiple cellular targets, resulting in antiangiogenic effects, apoptosis induction, tumor growth inhibition, metastasis prevention, and enhanced antitumor immunity. Pre-clinical studies have demonstrated its effectiveness against various sarcoma cell lines. Clinical trials have evaluated regorafenib in patients with different sarcoma subtypes, showing improved progression-free survival in leiomyosarcomas, synovial sarcomas, and other sarcomas. The safety profile of regorafenib in sarcoma trials is consistent with that in other tumor types, with hypertension and hand-foot skin reaction being the most common side effects. Further research is needed to determine its efficacy and safety in non-GIST sarcomas.

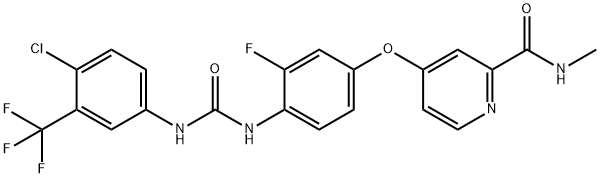
Figure 1. Regorafenib
Regorafenib in patients with non-GIST sarcomas
Regorafenib is a multikinase inhibitor that targets several cellular targets, including VEGFR, KIT, PDGFR, and FGFR. This results in an antiangiogenic effect, induces apoptosis, inhibits tumor growth, prevents metastases, and enhances antitumor immunity. Regorafenib has been clinically effective and is approved for the treatment of advanced colorectal cancer, advanced GIST, and hepatocellular cancer. In pre-clinical studies, regorafenib has demonstrated broad anti-tumor activity, including against Ewing sarcoma, osteosarcoma, and rhabdomyosarcoma cell lines. Regorafenib was able to inhibit tumor cell growth in patient-derived xenograft models of these three pediatric cancers, as well as a model of doxorubicin-resistant Ewing sarcoma. These findings suggest that regorafenib may have potential in treating patients with non-GIST sarcomas. Further clinical trials are needed to determine the safety and efficacy of regorafenib in this patient population. Overall, regorafenib's inhibition of various cellular targets makes it a promising candidate for the treatment of a variety of cancers, including non-GIST sarcomas. 1
Clinical trials for the treatment of sarcomas
Regorafenib has been evaluated for the treatment of non-GIST sarcomas in six clinical trials, including various sarcoma subtypes of both STS and bone sarcomas. One of these trials, REGOSARC, was a phase 2, randomized, comparative, placebo-controlled trial in adult patients with advanced STS who had received previous doxorubicin or other anthracycline treatment. The primary endpoint was PFS as per RECIST v1.1. The results showed that patients with leiomyosarcomas, synovial sarcomas, and other sarcomas treated with regorafenib had longer PFS when compared with placebo-treated patients. Another trial, SARC024, was a phase 2 trial evaluating regorafenib in patients with refractory sarcoma. The primary endpoint was also PFS as assessed by investigators as per RECIST v1.1. Patients who had undergone prior systemic therapy with a small molecule kinase inhibitor were excluded. Regorafenib was also evaluated in a phase 1/1b study in pediatric patients with recurrent or refractory solid tumors, including rhabdomyosarcoma and Ewing sarcoma. The primary endpoint was safety, and a dose expansion study was initiated in which regorafenib is being administered in combination with intravenous vincristine and irinotecan. Overall, regorafenib has shown promising results in the treatment of sarcomas, particularly in certain sarcoma subtypes. 2
Safety in sarcoma clinical trials
The safety of regorafenib in clinical trials for advanced sarcomas has been consistent and comparable to its safety profile in patients with other tumor types, such as GIST, CRC, and HCC. Grade ≥3 treatment-emergent adverse events (TEAEs) commonly associated with regorafenib treatment include hypertension and hand and foot skin reaction (HFSR). The incidence of grade ≥3 hypertension ranged from 10% to 24%, while the incidence of grade ≥3 HFSR ranged from 5% to 17% in published sarcoma trials. In these trials, a similar proportion of patients required regorafenib dose reductions and dose interruptions due to TEAEs, ranging from 38% to 69% and 35% to 59%, respectively. Only 11% of patients permanently discontinued regorafenib treatment in one trial, while no patients discontinued treatment in other trials. Based on clinical experience, recommendations have been made to prevent, monitor, and proactively manage common TEAEs such as fatigue, HSFR, rash, stomatitis, and diarrhea. Fatigue may require dose reductions if severe but often improves in subsequent treatment cycles. HSFR, characterized by localized hyperkeratotic lesions, is the most common toxicity leading to dose reductions and should be closely monitored and managed promptly. 3
Reference
1. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 734506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255.
2. Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, Chevreau C, Piperno-Neumann S, Bompas E, Salas S, Perrin C, Delcambre C, Liegl-Atzwanger B, Toulmonde M, Dumont S, Ray-Coquard I, Clisant S, Taieb S, Guillemet C, et al Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(12):1732–1742.
3. Blay JY, Duffaud F, George S, Maki RG, Penel N. Regorafenib for the Treatment of Sarcoma. Curr Treat Options Oncol. 2022;23(11):1477-1502.
You may like
Related articles And Qustion
Lastest Price from Regorafenib manufacturers

US $0.00/KG2025-04-21
- CAS:
- 755037-03-7
- Min. Order:
- 10g
- Purity:
- 99.5%min
- Supply Ability:
- 10kg

US $0.00/kg2025-03-07
- CAS:
- 755037-03-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons