Dimethocaine: Exploring the Metabolic Pathways of a Synthetic Cocaine Derivative
General Description
Dimethocaine is a synthetic cocaine derivative identified for its local anesthetic and central nervous system effects, particularly inhibiting dopamine reuptake. Initial studies on its metabolism by cytochrome P450 enzymes (P450s) highlighted the significant roles of P450 1A2, 2C19, 2D6, and 3A4 in its biotransformation, with P450 3A4 and P450 2D6 being crucial for its hepatic clearance. Additionally, NAT2 was found to be the key enzyme responsible for DMC's N-acetylation, a process not effectively catalyzed by NAT1. This metabolic understanding emphasizes the potential for individual pharmacokinetic variations due to enzyme polymorphism, which could affect DMC's safety profile and necessitates further research to fully grasp these implications.

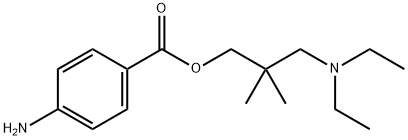
Figure 1. Dimethocaine
Overview
Dimethocaine, also known as DMC or larocaine, was originally marketed in the 1930s as a local anesthetic for dental and ophthalmological use. Its chemical structure is 3-diethylamino-2,2-dimethylpropyl-4-aminobenzoate. Beyond its anesthetic properties, Dimethocaine affects the central nervous system by inhibiting dopamine reuptake, similar to the action of cocaine. This similarity has led to its classification as a "synthetic cocaine derivative" by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) in 2013. Its recreational use is noted for inducing euphoria and relaxation, albeit with negative aftereffects like severe hangovers and fatigue. Comparative studies in rats have demonstrated that Dimethocaine's effectiveness as a dopamine-reuptake inhibitor is nearly equivalent to that of cocaine, particularly in its affinity for the dopamine transporter in the nucleus accumbens, a key region in the brain's reward system. This pharmacological profile underscores the potential for abuse and the need for careful monitoring and regulation. 1
In vitro metabolism catalyzed by P450s
Studies on the in vitro metabolism of Dimethocaine catalyzed by cytochrome P450 enzymes (P450s) reveal intricate details about its biotransformation. Initial screenings with the ten most abundant human hepatic P450s and human liver microsomes (HLM) identified key enzymes involved in Dimethocaine's metabolism, primarily focusing on N-deethylation and hydroxylation at the p-aminobenzoic acid part of the molecule. Kinetic parameters were successfully determined for P450 1A2, 2C19, 2D6, and 3A4, indicating their significant role in metabolizing Dimethocaine, whereas enzymes like P450s 2B6, 2C8, 2C9, and 3A5 showed minimal activity. The study utilized Michaelis–Menten kinetics to understand the enzyme-substrate interactions, revealing P450 3A4 as predominantly responsible for deethylation and P450 2D6 for hydroxylation processes. This suggests that P450 3A4 and P450 2D6 are crucial for the hepatic clearance of Dimethocaine, similar to findings with related compounds like lidocaine and bupivacaine. The potential for clinically relevant interactions between Dimethocaine and inhibitors of these P450 enzymes, such as macrolide antibiotics (P450 3A4 inhibitors) and SSRIs (P450 2D6 inhibitors), appears limited due to the involvement of multiple CYP catalyzed steps and different isoforms in Dimethocaine's metabolism, indicating a complex but discernible metabolic pathway for this compound. 2
In vitro metabolism catalyzed by NAT2
The metabolism of Dimethocaine, particularly its N-acetylation process, has been closely studied in relation to the NAT1 and NAT2 isozymes. Given Dimethocaine's p-aminobenzoic acid structure, initial hypotheses leaned towards NAT1 playing a significant role. However, it was discovered that only NAT2 is capable of catalyzing Dimethocaine's N-acetylation effectively. This conclusion was drawn from enzyme kinetic studies that showed negligible product formation by NAT1, whereas NAT2 displayed a clear capacity for this metabolic pathway, as evidenced by its kinetic profile fitting the Michaelis–Menten model. The specific kinetic parameters for Dimethocaine acetylation by NAT2 were determined, with a Km value of 102 µM and a Vmax of 1.1 units/min/pmol, highlighting NAT2's efficiency in processing Dimethocaine. This insight into DMC's metabolism underscores the importance of understanding enzyme polymorphism, especially since NAT1 and NAT2 exhibit high variability among individuals. Such genetic differences can significantly impact the biotransformation of aromatic amines, potentially leading to varied pharmacokinetic outcomes and increased risk of adverse effects, including carcinogenicity. This aspect is particularly crucial for populations with a slow acetylation phenotype, which encompasses around 50% of Europeans. These findings suggest that individual variations in NAT2 activity could influence Dimethocaine's plasma levels and the presence of its acetylated metabolites in urine, warranting further research to fully understand these dynamics and their clinical implications. 2
Reference
1. Graham JH 3rd, Maher JR, Robinson SE. The effect of cocaine and other local anesthetics on central dopaminergic neurotransmission. J Pharmacol Exp Ther. 1995;274(2):707-717.
2. Meyer MR, Lindauer C, Maurer HH. Dimethocaine, a synthetic cocaine derivative: studies on its in vitro metabolism catalyzed by P450s and NAT2. Toxicol Lett. 2014;225(1):139-146.
Related articles And Qustion
Lastest Price from Dimethocaine manufacturers

US $10.00/ASSAYS2025-05-04
- CAS:
- 94-15-5
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00-0.00/Kg2025-04-21
- CAS:
- 94-15-5
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons