Dimethocaine:Application, Activity, Pharmacokinetics and Toxicity
General Description
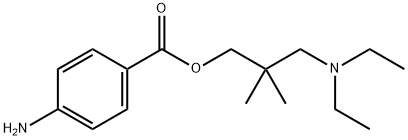
Dimethocaine (DMC, larocaine, 3-diethylamino-2,2-dimethylpropyl)-4-aminobenzoate) was marketed as local anesthetic in the 1930s and used in dentistry and ophthalmology.[1]It is a synthetic product, is the p-aminobenzoyl ester of 2,2-dimethyl-3-diethylamino-1-propanol. It is supplied in the form of a hydrochloride, a white cryst. powder sol. in water up to 50%. Chemically it is related to procaine-HCl and tutocaine.[2]
Besides local anesthetic effects, dimethocaine has also effects on the central nervous system acting as dopamine-reuptake-inhibitor. It is listed by the EMCDDA under the category “synthetic cocaine derivatives”.The abuse is described to produce feelings of euphoria and sometimes relaxed feeling accompanied with side effect such as a strong hangover and fatigue. Dimethocaine was shown to have high affinity for the dopamine transporter mainly in the nucleus accumbens stimulating the reward system. Furthermore, the previous litertaures have shown that dimethocaineis nearly as potent as cocaine concerning the dopamine-reuptake-inhibitor efficiency.Dimethocaine, a synthetic derivative of cocaine, is distributed and consumed as “new psychoactive substance” (NPS).It is mainly metabolized by N-acetylation, N-deethylation or hydroxylation.The endogenous N-acetylation as main part of dimethocaine metabolism was catalyzed by the NAT2 isozyme.[1]

Figure 1 Dimethocaine powder
Application
The dimethocaine has cocaine-like effects. For example, dimethocaine was found to be nearly as potent as cocaine in blocking dopamine uptake in striatal synaptosomes and that it competed for binding to the transporter. Also, as mentioned previously, dimethocaine was shown to be consistently self-administered at a high rate by monkeys and to substitute fully for cocaine in rats and monkeys trained to discriminate cocaine from vehicle. What’s more, dimethocaine has also been shown to induce rotational behavior in substantia nigra-lesioned rats. The present findings showing a stimulant-like profile of dimethocaine are in agreement with the notion that the reinforcing and the activating effects of psychostimulants are mediated by a common mechanism.[4]In addition, dimethocaine, local anesthetics of amide and ester classes, respectively, showed effective antinociceptive responses in all tests at nontoxic doses and these responses are mediated, at least in part, by their central actions.[5]
Activity
On the one hand, as expected for a central nervous system (CNS) stimulant, dimethocaine acts mainly as a dopaminereuptake-inhibitor. Like cocaine, dimethocaine is snorted since oral ingestion would lead to rapid hydrolysis of dimethocaine.On the other hand,dimethocaine is similar potent as cocaine concerning the DA-reuptake-inhibitor efficiency, and it has high affinity for the DA transporter mainly in the nucleus accumbens stimulating the reward system.[3]
Pharmacokinetics
Initial activity screening revealed that only NAT2 was capable catalyzing the N-acetylation of dimethocaine. Besides the extensive N-acetylation of the p-aminobenzoic acid part of the parent compound, most of the phase I metabolites were excreted as N-acetyl derivatives.It is obvious that there might be individual pharmacokinetic differences of the endogenous N-acetylation of dimethocaine, which may lead to increased negative side effects such as cardiotoxicity. Such interactions may be much more important for people with a slow acetylation phenotype. To what extent these points affect the plasma concentrations of dimethocaine and also the concentration of acetylated dimethocaine metabolites in urine.the presented metabolism study demonstrated the extensive metabolism of dimethocaine by the rat mainly via hydroxylation and deethylation as well as acetylation and glucuronidation. The endogenous N-acetylation as main part of dimethocaine metabolism was catalyzed only by the NAT2 isozyme. [3]
Toxicity
After consumption of dimethocaine, consumers describe feelings of euphoria but also dyspnea and complaints of angina pectoris. The intravenous abuse is described to produce an initial flush and an alert or sometimes even relaxed feeling in the first minutes after injection accompanied with side effect such as a strong hangover and fatigue.So, it is careful for its usage.[3]
References
[1]Meyer MR,et.al. Dimethocaine, a synthetic cocaine derivative: studies on its in vitro metabolism catalyzed by P450s and NAT2[J]. Toxicology Letters. 2014, 225(1): 139-146.
[2]LEO L,MAYER MD.LAROCAINE, A NEW ANESTHETIC[J]. Archives of Ophthalmology, 1935, 14(3): 408-411.
[3]Lindauer C. Toxicokinetics of Emerging Drugs of Abuse: In vivo and in vitro studies on the metabolic fate of the cocaine-derived designer drug dimethocaine[J]. 2014.
[4]Rigon, AR., Takahashi, RN.Stimulant activities of dimethocaine in mice: reinforcing and anxiogenic effects[J].Psychopharmacology. 1996,127:323-327.
[5]Rigon AR, Takahashi RN. The effects of systemic procaine, lidocaine and dimethocaine on nociception in mice[J]. General pharmacology, 1996, 27(4): 647-650.
You may like
Related articles And Qustion
See also
Lastest Price from Dimethocaine manufacturers

US $10.00/ASSAYS2025-05-04
- CAS:
- 94-15-5
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00-0.00/Kg2025-04-21
- CAS:
- 94-15-5
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons