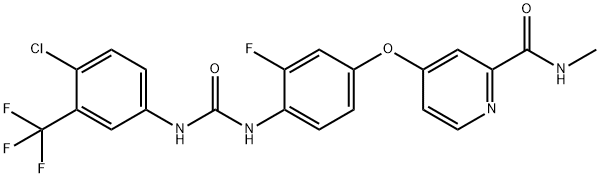
Regorafenib: Applications, metabolism, pharmacokinetics and toxicity
General description
Regorafenib is an oral diphenylurea multi-kinase inhibitor that targets angiogenic (VEGFR1-3, TIE2), stromal (PDGFR-b, FGFR), and oncogenic receptor tyrosine kinases (KIT, RET, and RAF). Regorafenib is the first small-molecule multi-kinase inhibitor to achieve survival benefits in metastatic colorectal cancer that has progressed after all standard therapies. Consequently, Regorafenib was FDA-approved for this indication in 2012. In addition, Regorafenib treatment resulted in a significant improvement in progression-free survival (PFS) compared to placebo in patients with metastatic gastrointestinal stromal tumors (GIST) after progression on standard treatments and is also FDA-approved in this indication since 2013. In 2017, Regorafenib has been FDA-approved for the treatment of patients with advanced hepatocellular carcinoma (HCC) previously treated with Sorafenib. Regorafenib significantly improved PFS and overall survival (OS) compared to placebo in this situation. Regorafenib has also been examined in several clinical trials (mostly phase II) in different tumor entities, including renal cell carcinoma (RCC), soft-tissue sarcoma (STS), and additional phase II trials ongoing. Its appearance is as follows:

Figure 1 Appearance of Regorafenib
Applications
Regorafenib inhibits VEGF receptors 1, 2, and 3 and PDGF receptors like other antiangiogenic tyrosine kinase inhibitors approved for the treatment of advanced renal cell cancer. Regorafenib also inhibits other potentially important angiogenic kinases like TIE2, the activation of which is thought to be important in tumor escape mechanisms. In 2017, Regorafenib has been approved by the FDA for the treatment of patients with advanced HCC who progressed on Sorafenib. This decision was based on the final data of the RESOURCE trial [1].
Mechanism of Action
Regorafenib is a small-molecule inhibitor of various membrane-bound and intracellular kinases involved in normal cellular functions as well as pathologic processes, such as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment. In biochemical in vitro or cell-based assays, Regorafenib or its major human active metabolites M-2 and M-5 inhibited the activity of RET, VEGFR 1-3, KIT, PDGFR-alpha, PDGFR-beta, FGFR1, FGFR2, TIE2, DDR2, TrkA, Eph2A, RAF-1, BRAF, BRAFV600E, SAPK2, PTK5, and Abl at concentrations that can be achieved clinically.
Pharmacokinetics
The standard dose of Regorafenib is 160 mg taken orally once daily as tablets. The mean relative bioavailability of orally taken Regorafenib is 69%. Regorafenib undergoes enterohepatic circulation with multiple plasma concentration peaks observed across the 24-h dosing interval. Regorafenib is highly bound (99.5%) to human plasma proteins and metabolized by CYP3A4 and UGT1A9. The main circulating metabolites of Regorafenib in human plasma are M-2 (N-oxide) and M-5 (N-oxide and N-desmethyl). Both metabolites have similar in vitro pharmacological activity and are highly protein bound (>99.0%). The elimination half-life for Regorafenib and its M-2 metabolite in plasma is 28 and 25 h, respectively. M-5 has a longer elimination half-life of 51 h. Approximately, 71% of a radiolabeled dose of Regorafenib is excreted via feces and 19% of the dose is excreted via urine. Based on a population pharmacokinetic analysis, there is no clinically relevant effect of age, gender, or weight on the pharmacokinetics of Regorafenib.
Toxicity
In the CORRECT trial [2] (mCRC, 760 patients), Regorafenib caused adverse reactions involving the skin and subcutaneous tissues (72% vs. 24%) including hand–foot skin reaction (HFSR) and severe rash requiring dose modification. Serious adverse skin reactions including erythema multiforme (0.2% vs. 0%) and Stevens–Johnson syndrome (0.2% vs. 0%) were more frequent in Regorafenib-treated patients. Toxic epidermal necrolysis occurred in 0.17% of 1200 Regorafenib-treated patients across all clinical trials. In a meta-analysis [3], 1078 patients treated with Regorafenib for mCRC, GIST, renal cell carcinoma (RCC), and hepatocellular carcinoma (HCC) were included. The overall incidence of all-grade and high-grade HFSR were 60.5 and 20.4%, respectively. The relative risk (RR) of all-grade and high-grade HFSR with Regorafenib compared to controls was increased for all-grade (RR = 5.4) and high-grade (RR = 41.99) HFSR. Interestingly, the incidence of HFSR varied significantly with tumor type (p = 0.007), and was 71.4% in RCC, 60.2% in GIST, 50.0% in HCC, and 46.6% in mCRC, respectively.
References
[1]Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66
[2]Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312
[3]Belum VR, Wu S, Lacouture ME (2013) Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs 31(4):1078–1086
You may like
Related articles And Qustion
See also
Lastest Price from Regorafenib manufacturers

US $0.00/KG2025-04-21
- CAS:
- 755037-03-7
- Min. Order:
- 10g
- Purity:
- 99.5%min
- Supply Ability:
- 10kg

US $0.00/kg2025-03-07
- CAS:
- 755037-03-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons