Pyrroloquinoline Quinone: Synthesis and Biology in Antimicrobial Effects and Neuroprotection
General Description
Pyrroloquinoline quinone is a unique molecule with diverse biological implications. Synthetic efforts have focused on creating the tricyclic pyrroloquinolinedione scaffold using different retrosynthetic pathways. Pyrroloquinoline quinone has demonstrated antimicrobial effects, inhibiting the growth of various pathogens and holding potential for drug discovery and plant protection. Additionally, Pyrroloquinoline quinone has shown neuroprotective properties, protecting against neurotoxic substances, enhancing spatial memory, and reducing memory impairment in animal models. These findings suggest that Pyrroloquinoline quinone could be a valuable therapeutic agent for combating bacterial infections and neurodegenerative disorders.

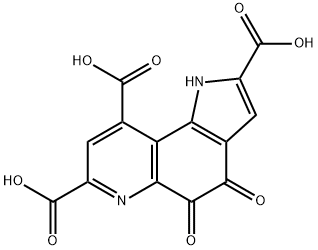
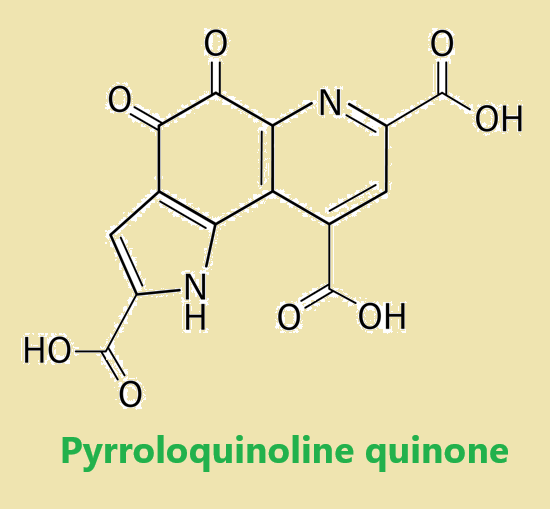
Figure 1. Pyrroloquinoline quinone
Synthesis
Pyrroloquinoline quinone is a unique heterocyclic molecule with interesting biological implications, which has stimulated synthetic efforts to create the tricyclic pyrroloquinolinedione scaffold. There are three alternative retrosynthetic pathways to achieve this structure: (i) sequential creation of two heterocyclic units onto the aromatic core, with construction of either ring A or ring C first, (ii) a convergent approach in which rings A and C are coalesced to form ring B, and (iii) an alternative convergent strategy in which ring A is added to a preformed indole precursor which evolves as rings B and C. The initial synthetic efforts on Pyrroloquinoline quinone were reported by Corey's and Weinreb's groups in 1981, and modifications were made to prepare isotopically labeled forms of Pyrroloquinoline quinone. The Buchi synthesis represented a retrobiosynthetic strategy in which the two complete carbon and nitrogen frameworks were united in the second synthetic step and then elaborated. The shortest and highest yielding synthesis was the four-step sequence developed by Boger. Overall, these various synthetic approaches provide a diverse toolkit for the preparation of Pyrroloquinoline quinone and its derivatives. 1
Biology in Antimicrobial Effects and Neuroprotection
Antimicrobial Effects
The antimicrobial effects of Pyrroloquinoline quinone have been demonstrated in various studies. One such study focused on the bacterium Rahnella aquatilis HX2, which is a human pathogen but also used as a biocontrol agent. When the Pyrroloquinoline quinone biosynthesis genes pqqA and pqqB were mutated, no Pyrroloquinoline quinone or its precursor, gluconic acid (9), was produced. This resulted in reduced antibacterial activity against sunflower crown gall disease and a loss of ability to solubilize mineral phosphate. Another example is Enterobacter intermedium 60-2G, a phosphate-solubilizing bacterium that provides systemic resistance to the plant pathogen Erwinia carotovora through the production of gluconic acid (9). Mutation of the Pyrroloquinoline quinone biosynthesis genes in this organism also abolished the synthesis of Pyrroloquinoline quinone, leading to the loss of hydroxyapatite solubilization and inhibition of rice pathogen Magnaporthe grisea KI-409 and E. carotovora. Interestingly, a bioinformatics search revealed 47 putative Pyrroloquinoline quinone-producing organisms that are known pathogenic organisms for humans, other mammals, or plants. This discovery holds potential for future drug discovery and plant protection initiatives. For example, the Gram-negative bacterium Pantoea ananatis (formerly Erwinia ananis) is an emerging plant and human pathogen with homologues of glucose dehydrogenase and a possible Pyrroloquinoline quinone biosynthetic operon. Targeting the Pyrroloquinoline quinone gene operon could be a significant therapeutic approach. In summary, the antimicrobial effects of Pyrroloquinoline quinone have been observed in various organisms, and understanding the biosynthesis and regulation of Pyrroloquinoline quinone production can provide valuable insights for combating bacterial infections and protecting plants from pathogens. 1
Neuroprotection
The neuroprotective effects of Pyrroloquinoline quinone have been extensively studied in various test systems. Pyrroloquinoline quinone has demonstrated its ability to protect against toxicity induced by rotenone and methylmercury, two neurotoxic substances. It also reduces the negative effects of carotid ligation in rats and transient cerebral artery occlusion. Furthermore, Pyrroloquinoline quinone has shown promising results in preventing α-synuclein fibril formation, a key pathological feature in neurodegenerative diseases such as Parkinson's disease. Prophylactic administration of Pyrroloquinoline quinone has been found to enhance spatial memory following traumatic brain injury. In cellular models, Pyrroloquinoline quinone has been shown to protect neuroblastoma SH-SY5Y cells from oxidative stress induced by substances like 6-hydroxydopamine or H2O2. In animal models, Pyrroloquinoline quinone supplementation has been found to improve learning and memory, even in the presence of oxidative stress. Additionally, a combination treatment of clozapine and Pyrroloquinoline quinone in rats with MK801-induced schizophrenia has been shown to reduce memory impairment. This effect is attributed, at least in part, to the NMDA binding effect of Pyrroloquinoline quinone. Overall, Pyrroloquinoline quinone exhibits significant neuroprotective properties across different experimental settings, suggesting its potential therapeutic value for neurodegenerative disorders and cognitive dysfunction. 2
Reference
1. Cordell GA, Daley SK. Pyrroloquinoline Quinone Chemistry, Biology, and Biosynthesis. Chem Res Toxicol. 2022;35(3):355-377.
2. Zhou X, Cai G, Mao S, et al. Modulating NMDA receptors to treat MK-801-induced schizophrenic cognition deficit: effects of clozapine combining with PQQ treatment and possible mechanisms of action. BMC Psychiatry. 2020;20(1):106.
Related articles And Qustion
Lastest Price from Pyrroloquinoline quinone manufacturers

US $0.00-0.00/kg2025-08-19
- CAS:
- 72909-34-3
- Min. Order:
- 1kg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 2tons

US $0.00-0.00/g2025-05-21
- CAS:
- 72909-34-3
- Min. Order:
- 100g
- Purity:
- 99%
- Supply Ability:
- 50kg