Pyridinium p-Toluenesulfonate: properties, applications in organic synthesis and safety
General Description
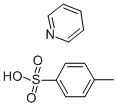
Pyridinium p-Toluenesulfonate (PPTS) is a white to off-white crystalline powder with various physical and chemical properties. It is highly soluble in polar solvents but has limited solubility in nonpolar solvents. PPTS is stable and can withstand moderate temperatures without significant degradation. It acts as a mild acid catalyst, facilitating reactions such as esterifications and rearrangements. In water, PPTS imparts acidity with a pH around 1 to 2. With a molecular weight of approximately 295.35 g/mol, it is odorless and convenient for laboratory handling. PPTS is used as a catalyst in important synthesis steps, including Claisen rearrangements, acetal deprotection, diastereoselective reactions, and regioselective additions. Proper safety precautions should be followed when handling PPTS, including wearing protective equipment and avoiding ingestion or inhalation.

Figure 1. Pyridinium p-Toluenesulfonate
Properties
Pyridinium p-Toluenesulfonate is a versatile chemical compound with various physical and chemical properties. It appears as a white to off-white crystalline powder that is often odorless. PPTS is highly soluble in polar solvents like water, methanol, ethanol, and acetone but has limited solubility in nonpolar solvents. Its melting point ranges from approximately 165°C to 170°C, depending on purity. PPTS is stable under normal conditions and can withstand moderate temperatures without significant decomposition or degradation. While it is not hygroscopic, prolonged exposure to moisture should be avoided. PPTS acts as a mild and selective acid catalyst, facilitating reactions like esterifications, acetalizations, and Beckmann rearrangements. In water, PPTS imparts acidity with a pH ranging from around 1 to 2 due to its sulfonic acid group. With a molecular weight of approximately 295.35 grams per mole, PPTS is considered odorless, making it convenient for laboratory handling. 1
Applications in organic synthesis
Pyridinium p-Toluenesulfonate is a catalyst that plays a crucial role in several key steps of the synthesis. It acts as a Lewis acid catalyst, facilitating the Claisen rearrangement, one-pot acetal deprotection, diastereoselective Henry reaction, and regioselective azidoalkoxylation of enol ether. The Claisen rearrangement involves the isomerization of a vinyl ether to form an allyl vinyl ether. PPTS catalyzes this reaction, allowing for structural changes and the formation of reactive intermediates. PPTS is also responsible for the one-pot acetal deprotection, where it facilitates the removal of protecting groups from acetals, generating aldehydes or ketones. This step exposes reactive functional groups for further transformations. In the diastereoselective Henry reaction, PPTS catalyzes the addition of a nitro group to aldehydes or ketones, resulting in the formation of β-nitroalcohols. It is important for constructing the required trans ring junction and controlling the stereochemistry of the final product. Additionally, PPTS is involved in the CAN-mediated regioselective azidoalkoxylation of enol ether. It facilitates the addition of an azide group to enol ether substrates, forming azidoalkoxy products. The regioselectivity of this reaction is controlled by PPTS and can be influenced by factors such as temperature and solvent choice. Overall, Pyridinium p-Toluenesulfonate acts as a versatile catalyst in the synthesis, enabling efficient construction of desired structural motifs and selective functional group transformations. Its involvement in the Claisen rearrangement, one-pot acetal deprotection, diastereoselective Henry reaction, and regioselective azidoalkoxylation highlights its importance in the synthetic process. 2
Safety
Pyridinium p-Toluenesulfonate is a chemical compound that can be safely handled and used when proper safety precautions are followed. It is important to handle PPTS in a well-ventilated area, wearing appropriate personal protective equipment (PPE) such as gloves, safety glasses, and a lab coat. PPTS should be stored in a cool, dry place away from ignition sources. Inhalation and skin contact should be avoided, and in case of accidental exposure, affected areas should be rinsed with water and medical attention sought if necessary. Eye contact should be treated by rinsing eyes with flowing water and seeking medical assistance. PPTS should not be ingested, and any accidental ingestion should be immediately addressed by seeking medical help. Proper disposal methods should be followed in accordance with local regulations. It is crucial to consult the material safety data sheet (MSDS) for specific safety information and guidelines provided by the manufacturer. These measures ensure the safe handling and use of Pyridinium p-Toluenesulfonate in various applications. 3
Reference
1. PubChem: Pyridinium p-toluenesulfonate. National Library of Medicine, 2005, CID: 466102.
2. Chavan SP, Kadam AL, Lasonkar PB, Gonnade RG. Synthesis of 3-Azidopiperidine Skeleton Employing Ceric Ammonium Nitrate (CAN)-Mediated Regioselective Azidoalkoxylation of Enol Ether: Total Synthesis of D2 Receptor Agonist (±)-Quinagolide. Org Lett, 2018, 20(22):7011-7014.
3. SAFETY DATA SHEET: Pyridinium p-toluenesulfonate. Thermo Fisher SCIENTIFIC, 2010, Cat No.: AC213740000.
You may like
Related articles And Qustion
See also
Lastest Price from Pyridinium p-Toluenesulfonate manufacturers

US $0.00/kg2025-09-26
- CAS:
- 24057-28-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200tons
US $10.00/KG2025-04-21
- CAS:
- 24057-28-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt