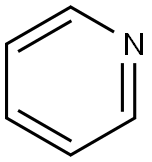
Pyridine-Health Hazards and Toxicity
Pyridine is a clear, colorless to pale yellow, flammable liquid with a sharp, penetrating, nauseating fish-like odor. Odor threshold concentrations in water and air were 2 ppm (Buttery et al., 1988) and 21 ppbv (Leonardos et al., 1969), respectively. Detection odor threshold concentrations of 0.74 mg/m3 (2.3 ppmv) and 6 mg/m3 (1.9 ppmv) were experimentally determined by Katz and Talbert (1930) and Dravnieks (1974), respectively. Cometto-Muiz and Cain (1990) reported an average nasal pungency threshold concentration of 1,275 ppmv.
Toxicity Data
LD50 oral (rat) 891 mg/kg
LD50 skin (rabbit) 1121 mg/m3
PEL (OSHA) 5 ppm (15 mg/m3)
TLV-TWA (ACGIH) 5 ppm (15 mg/m3)
Hazards Identification
Acute oral toxicity Category 4 (H302)
Skin Corrosion/irritation Category 1 B (H314)
Serious Eye Damage/Eye Irritation Category 1 (H318)
Respiratory Sensitization Category 1 (H334)
Major Hazards
Highly flammable liquid
Toxicity
The acute toxicity of pyridine is low. Inhalation causes irritation of the respiratory system and may affect the central nervous system, causing headache, nausea, vomiting, dizziness, and nervousness. Pyridine irritates the eyes and skin and is readily absorbed, leading to systemic effects. Ingestion of pyridine can result in liver and kidney damage. Pyridine causes olfactory fatigue, and its odor does not provide adequate warning of the presence of harmful concentrations.
Pyridine has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans. Chronic exposure to pyridine can result in damage to the liver, kidneys, and central nervous system.
Flammability and Explosibility
Pyridine is a highly flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance and "flash back." Pyridine vapor forms explosive mixtures with air at concentrations of 1.8 to 12.4% (by volume). Carbon dioxide or dry chemical extinguishers should be used for pyridine fires.
Reactivity and Incompatibility
Pyridine may react violently with dinitrogen tetroxide, acid chlorides and anhydrides, perchloric acid, and strong oxidizing agents.
Storage and Handling
Pyridine should be handled in the laboratory using the "basic prudent practices". In particular, pyridine should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If pyridine is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, remove all ignition sources, soak up the pyridine with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Excess pyridine and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
You may like
Related articles And Qustion
See also
Lastest Price from Pyridine manufacturers
US $1.00/kg2025-04-21
- CAS:
- 110-86-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $1.10/g2025-04-17
- CAS:
- 110-86-1
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min