Potassium hydride-Health Hazards and Toxicity
A white orgreyish white crystalline solid, KH;r.d. 1.43–1.47. It is prepared by passinghydrogen over heated potassiumand marketed as a light grey powderdispersed in oil. The solid decomposeson heating and in contact withmoisture and is an excellent reducingagent. Potassium hydride is a firehazard because it produces hydrogenon reaction with water.
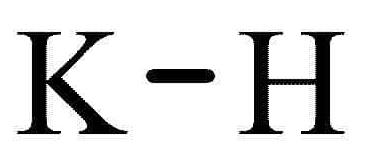
Major Hazards
Reacts violently with water, liberating highly flammable hydrogen gas; causes severe burns on eye or skin contact.
Toxicity
Sodium hydride and potassium hydride react with the moisture on skin and other tissues to form highly corrosive sodium and potassium hydroxide. Contact of these hydrides with the skin, eyes, or mucous membranes causes severe burns; thermal burns may also occur due to ignition of the liberated hydrogen gas.
Fire Hazard
Potassium hydride is flammable solid that ignite on contact with moist air. Potassium hydride presents a more serious fire hazard than sodium hydride. The mineral oil dispersions do not ignite spontaneously on exposure to the atmosphere. Sodium hydride and potassium hydride fires must be extinguished with a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay or graphite, or "Met-L-X" type solids. Water or CO2 extinguishers must never be used on sodium and potassium hydride fires.
Flammability and Explosibility
Potassium hydride and sodium hydride are flammable solids that ignite on contact with moist air. Potassium hydride presents a more serious fire hazard than sodium hydride. The mineral oil dispersions do not ignite spontaneously on exposure to the atmosphere. Sodium hydride and potassium hydride fires must be extinguished with a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay or graphite, or "Met-L-X®" type solids. Water or CO2 extinguishers must never be used on sodium and potassium hydride fires.
Reactivity and Incompatibility
Potassium hydride and sodium hydride react violently with water, liberating hydrogen, which can ignite. Oil dispersions of these hydrides are much safer to handle because the mineral oil serves as a barrier to moisture and air. Potassium hydride may react violently with oxygen, CO, dimethyl sulfoxide, alcohols, and acids. Explosions can result from contact of these compounds with strong oxidizers. Potassium hydride is generally more reactive than sodium hydride.
Storage and Handling
Sodium hydride and potassium hydride should be handled in the laboratory using the "basic prudent practices", supplemented by the additional precautions for work with flammable and highly reactive substances. Safety glasses, impermeable gloves, and a fire-retardant laboratory coat should be worn at all times when working with these substances. These hydrides should be used only in areas free of ignition sources and should be stored preferably as mineral oil dispersions under an inert gas such as argon.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If potassium hydride or sodium hydride is ingested, obtain medical attention immediately. If sodium hydride dust is inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, remove all ignition sources, quench the metal hydride, whether burning or not, with a dry chemical extinguishing medium, sweep up, place in an appropriate container under an inert atmosphere, and dispose of properly. Respiratory protection may be necessary in the event of a spill or release in a confined area.
Disposal
Excess potassium or sodium hydride and waste material containing these substances should be placed in an appropriate container under an inert atmosphere, clearly labeled, and handled according to your institution's waste disposal guidelines. Experienced personnel can destroy small quantities of sodium hydride and potassium hydride by the careful dropwise addition of t-butanol or iso-propanol to a suspension of the metal hydride in an inert solvent such as toluene under an inert atmosphere such as argon. Great care must be taken in the destruction of potassium hydride because of its greater reactivity. The resulting mixture of metal alkoxide should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
You may like
See also
Lastest Price from Potassium hydride manufacturers

US $15.00-10.00/KG2021-07-02
- CAS:
- 7693-26-7
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton


