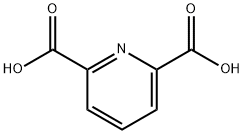
Pyridine-2,6-dicarboxylic Acid: A Versatile Compound Enhancing Metal Ion Analysis and Environmental Remediation
Pyridine-2,6-dicarboxylic acid (PDA) was employed as a lead-complexing agent. Batch testing involving PDA extraction of lead from spiked soils showed PDA to be an effective lead solubilizing agent across a wide pH range.

Metal Ion Analysis
Pyridine-2,6-dicarboxylic acid or dipicolinic acid (DPA) is known to form stable chelates with metal ions and oxometal cations and can display widely varying coordination demeanour functioning as a bidentate, tridentate, meridian or bridging ligand. DPA can stabilize unusual oxidation states and can form bridging hydrogen bonds due to its functional groups. It is a versatile N,O-chelating agent with limited stearic hindrance and can further provide possibility to form polymeric complexes through bridging coordination of carboxylates under suitable conditions. DPA can act as an interesting ligand due to its ability to form strong covalent bonds. Also the spatial separation of two carboxylate groups attached to the same aromatic ring leads to either a polymeric chain or a cyclo-oligomeric ring structure and there is a potential influence of nitrogen atom on the coordination mode.
Environmental Remediation
The biodegradability of 2,6-pyridine dicarboxylic acid has been used as a chelating agent for extraction of metal ions from contaminated soils. Initial experiments indicated that PDA was not biodegraded by unacclimated mixed cultures to any degree. However, acclimated mixed cultures degraded more than 80% of this compound within 12 h under different conditions without showing any sign of inhibition even at high concentrations up to 6 mM (1000 mg/l). The results of PDA biodegradation at all concentrations tested in aqueous solution were very similar to those observed in presence of soil slurry, except that a slight lag of microbial growth occurred at all PDA concentrations in the latter case.
References:
[1] SHANKHA K. BANERJI T P R. Biodegradation of the chelator 2,6-pyridine dicarboxylic acid (PDA) used for soil metal extraction[J]. Waste management, 1998, 18 5: 293-358. DOI:10.1016/S0956-053X(98)00077-4.[2] SADAF KHAN . Synthesis and characterization of transition metal 2,6-pyridinedicarboxylic acid derivatives, interactions of Cu(II) and Ni(II) complexes with DNA in vitro[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2009, 72 2: 229-454. DOI:10.1016/j.saa.2008.10.001.
[3] CHRIS S THOMAS. Pyridine-2,6-Dithiocarboxylic Acid and Its Metal Complexes: New Inhibitors of New Delhi Metallo β-Lactamase-1.[J]. Marine Drugs, 2020, 18 6. DOI:10.3390/md18060295.
[4] EDWARD MACAULEY Andrew H. Chelation extraction of lead from soil using pyridine-2,6-dicarboxylic acid[J]. Journal of Hazardous Materials, 1995, 40 3: 213-340. DOI:10.1016/0304-3894(94)00087-W.
You may like
Lastest Price from Pyridine-2,6-dicarboxylic acid manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 499-83-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 10 TONS

US $10.00/KG2025-04-21
- CAS:
- 499-83-2
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt