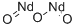Neodymium Oxide: Physicochemical Synthesis Methods and Cytotoxic Effects
General Description
Neodymium oxide (Nd2O3) is a rare earth oxide. It is a light blue-grey powder with a hexagonal crystal structure. It is almost insoluble in water and soluble in acid. Nd2O3 has unique properties such as thermal stability, hygroscopicity, good insulation properties and high dielectric constant, which are beneficial for industrial applications.

Figure 1. Neodymium oxide
Applications
Nd2O3 can be used to dope glass (including sunglasses), make solid-state lasers, and color glass and enamel. Glass doped with neodymium turns purple due to the absorption of yellow and green light and can be used in welding goggles. It is used as a starting material for the production of pure neodymium in organic synthesis; as a component for the preparation of high-temperature ceramics and superconductors; and as a catalyst for the oxidative coupling of methane, nitrogen decomposition, dehydrogenation of alcohols, and high-temperature processes.
In addition, it can also be used in the field of electrochemistry. The electrolytic production of metallic neodymium is carried out in molten neodymium fluoride salts containing neodymium oxide. Iron is used as a reaction anode in the electrolysis process to promote the electrochemical dissolution of iron in the melt and prevent the generation of PFC (perfluorocarbon) gas at the anode. Furthermore, the rare earth oxides are converted to rare earth fluorides using iron fluoride formed by the dissolution of iron. Thus, the fluorinating agent is constantly regenerated in situ, thus achieving continuous conversion of the neodymium oxide feed. The cathode product is a NdFe alloy, which can be used as a master alloy for the production of NdFeB magnets.
Synthesis Methods
Using simple neodymium salts as starting materials, neodymium oxide nanoprecursors in the form of fibrous or rod-like particles were successfully obtained under relatively low temperature hydrothermal conditions. The type of neodymium precursor determines the morphology of the final oxide as well as the crystal structure (hexagonal or cubic). Spherical Nd2O3 nanoparticles with a diameter less than 10 nm were directly prepared using the same synthesis method but at higher temperatures and longer times. Dip coating was used to prepare thermally stable neodymium oxide thin film coatings on different substrates.
Cytotoxic Effects
In a cytotoxicity study of Neodymium Oxide on liver cancer (HepG-2) and lung cancer (A-549) cells, Nd2O3 showed a moderate dose-dependent effect on cancer cells. ROS, cell cycle analysis, and qPCR demonstrated that Nd2O3 has the ability to cause cell death through ROS generation and genotoxicity pathways.
References:
[1] JAVED AHMAD . Neodymium oxide nanostructures and their cytotoxic evaluation in human cancer cells[J]. Journal of Trace Elements in Medicine and Biology, 2022, 73. DOI:10.1016/j.jtemb.2022.127029.See also
Lastest Price from Neodymium oxide manufacturers

US $90.00-2200.00/kg2025-06-09
- CAS:
- 1313-97-9
- Min. Order:
- 100kg
- Purity:
- 99.9%
- Supply Ability:
- 20MT

US $100.00/KG2025-04-21
- CAS:
- 1313-97-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt