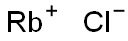Preparation and uses of Rubidium chloride
Description
Rubidium chloride is an alkali metal halide with the formula RbCl. It is a stable, white crystalline powder that is soluble in water and has a density of 2.8 g/mL at 25 °C. RbCl is hygroscopic and must be stored in isolation from atmospheric moisture, e.g. using desiccant. As an important rubidium compound, it is widely used in biomedicine, the electronics industry, and optical fields.
Preparation
Rubidium chloride is stable in air and exists in nature as a complex salt halite. It is mainly recovered from lithium mica, salt brine tailings processed by the lithium industry, and also extracted from rubidium-containing halide and rubidium-rich mineral water. The most common method for preparing pure rubidium chloride is to mix rubidium hydroxide with hydrochloric acid and then recrystallize and purify. Another method takes advantage of the balance of Na, NaCl, Rb, and RbCl at high temperatures. A expensive method is to react with rubidium metal and halogens:
2Rb(s)+ Cl2 (g)→ 2RbCl(s)
Uses
In the electronics industry, rubidium chloride can be used in the preparation of semiconductor materials and optoelectronic devices. In the field of optics, rubidium chloride can be used in the preparation of optical glass and optical fiber. In the biomedical field, rubidium chloride can be used to treat diseases such as heart disease and hypertension. It also acts as a radiotracer in nuclear medicine.
Rubidium is one of the trace elements in the human body. As early as the 1970s, rubidium chloride (RbCl) was reported to have neuroprotective effects. Hao et al. report the antiaging effect of RbCl in Caenorhabditis elegans, arguing that RbCl is a promising drug for anti-aging-related diseases. In particular, RbCl does increase the life span and enhance stress resistance in C. elegans without disturbing their fecundity. Interestingly, in 2019, it was found that RbCl can target Jnk/p38-mediated NF-κB activation to attenuate osteoclastogenesis while facilitating osteoblastogenesis both in vivo and in vitro. Therefore, RbCl may be used to coat the surface of biomaterials for orthopedic implants to prevent osteoporosis.
References
[1] Lv Y, et al. Selective extraction of cesium from high concentration rubidium chloride leach liquor of lepidolite. Desalination, 2022; 530: 115673.
[2] John S, et al. Hydration Structure at the Calcite–Water (10.4) Interface in the Presence of Rubidium Chloride. Langmuir, 2022; 38: 11691–11698.
[3] Hao M, et al. Rubidium Chloride Increases Life Span Through an AMPK/FOXO-Dependent Pathway in Caenorhabditis elegans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2021; 77: 1517–1524.
[4] Paschalis C, et al. Effects of Rubidium Chloride on the Course of Manic-Depressive Illness. Journal of the Royal Society of Medicine, 1978; 71.
[5] ;Ouyang ;Z, et al. Rubidium Chloride Targets Jnk/p38-Mediated NF-κB Activation to Attenuate Osteoclastogenesis and Facilitate Osteoblastogenesis. Frontiers in Pharmacology, 2019; 10.
You may like
Related articles And Qustion
See also
Lastest Price from Rubidium chloride manufacturers

US $30.00/kg2025-04-21
- CAS:
- 7791-11-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons

US $0.00-0.00/KG2025-04-15
- CAS:
- 7791-11-9
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg