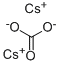
Application and Property of Cesium carbonate
Description
A variety of basic catalysts exist, such as alkaline carbonates, hydroxides, and fluorides. Among them, cesium carbonate is one of the most important alkaline carbonates. Due to its unique properties such as large cationic radius and easy solvation in a variety of organic solvents, cesium ions have attracted widespread attention in organic synthesis[1]. And Cs+ion is more deshielded and highly reactive.
Properties
Cesium carbonate is a colourless crystal or white powder that quickly absorbs moisture when placed in the air. Therefore, it should be sealed and dried when stored, and separately from acids. The aqueous solution of cesium carbonate is strongly alkaline, it can react with the acid to form the corresponding cesium salt and water and release carbon dioxide[2]. The chemical formula is Cs2CO3 and the melting point is 610 °C. At 20 ºC, its solubility is 261 g/100 mL. There are many preparation methods for it, and cesium salt is commonly used as raw material:
A. Add an appropriate amount of cesium chloride and excess nitric acid to the borosilicate glass container. After removing chlorine, the nitrate prepared by the reaction was poured into a platinum dish, and four times the amount of crystalline oxalic acid was added for the reaction to obtain oxalate. Then oxalate is roasted to obtain cesium carbonate.
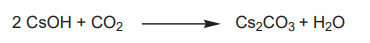
B. Prepared by the reaction of cesium hydroxide with carbon dioxide
2CsOH+CO2=Cs2CO3+H2O
Application
Cesium carbonate is a mild base, a unique feature that is necessary for selective deprotonation and its applicability to base-catalyzed reactions where it is less basic than hydroxides or alkoxides and stronger than other carbonates. Many of the properties exhibited by cesium carbonate in organic synthesis are derived from the softer Lewis acidity of cesium ions, which makes it soluble in organic solvents such as alcohols, DMF, and ether. Many organic transformations may require the presence of cesium carbonate to facilitate the deportation and carbon anion formation, as well as the arylation and alkylation of alcohols, carboxylic acids, and amines[3-4]. It can also be used for the formation of C-C bonds in reactions such as Michael. It can be used in the Alkylation and arylation of carbon, nitrogen, oxygen, and sulfur.
References
[1] Lehmann, et al. Cesium Carbonate (Cs2CO3). Synlett, 2004; 2447-2448.
[2] Yu M, et al.Expeditious synthesis of 9-allenylpurines via cesium carbonate catalyzed isomerization of 9-alkynylpurines. Phosphorus, Sulfur, and Silicon and the Related Elements, 2018; 193: 451-458.
[3] Rabie R, et al.Cesium carbonate as a mediated inorganic base in some organic transformations. Research on Chemical Intermediates, 2016; 43: 1979–2015.
[4] Fehr L, et al. Facile Multicomponent Synthesis of Oxazolidinones from Primary Amines and Cesium (Hydrogen)Carbonate. European Journal of Organic Chemistry, 2023; 26: e202300135.
You may like
Related articles And Qustion
See also
Lastest Price from Cesium carbonate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 534-17-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $192.00-7000.00/KG2025-04-21
- CAS:
- 534-17-8
- Min. Order:
- 1KG
- Purity:
- 97%
- Supply Ability:
- 1 ton