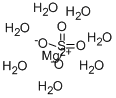
Preparation and Application of Magnesium sulfate heptahydrate
Magnesium sulfate heptahydrate (molecular formula MgSO4·7H2O), also known as sulfur bitter, bitter salt, Epsom salt, Epsom salt, is white or colorless needle-like or oblique columnar crystal, odorless, cool and slightly bitter, molecular weight: 246.47, The specific gravity is 1.68, easily soluble in water, slightly soluble in ethanol and glycerol, and soluble in its own crystal water at 67.5°C. Thermal decomposition, 70,80 ℃ is the loss of four molecules of crystal water. About 200 ℃ lose all crystal water into anhydrous. In the air (drying), it is easy to be weathered into powder, and when heated, the crystal water is gradually removed to become anhydrous magnesium sulfate. This product does not contain any toxic impurities.
Because magnesium sulfate heptahydrate is not easy to dissolve, it is easier to weigh than anhydrous magnesium sulfate, which is convenient for quantitative control in industry. It is mainly used in the manufacture of fertilizers, leather making, printing and dyeing, catalysts, papermaking, plastic materials, porcelain, pigments, matches, explosives and fireproof materials. It can be used for printing and dyeing thin cotton cloth and silk, as a weighting agent for cotton silk and a filler for kapok products; it is used as Epsom salt in medicine.

Preparation
Sulfuric acid method:
MgO+H2SO4+6H2O→MgSO4·7H2O
This method uses dolomite, serpentine, and bittern as raw materials. Add water or mother liquor into the neutralization reactor, and then slowly add sulfuric acid and bitter earth powder containing more than 85% magnesium oxide into the reactor according to a certain proportion to carry out neutralization reaction, control pH=5, and the concentration is 39-40 °Bé. Stir for 30min to make the reaction fully proceed. Its keeps the neutralization solution at 80℃, the filtered filtrate is sent to the crystallizer, and an appropriate amount of seed crystals are added, and then cooled, filtered, centrifuged, and dried at 50~55℃ , that is, magnesium sulfate. The mother liquor is returned for batching. The bittern obtained in the salt lake by the recrystallization method is naturally evaporated and concentrated into crude magnesium (crude magnesium sulfate). Using crude magnesium as raw material, add water to dissolve at 80~90℃, then clarify at 60~70℃, crystallize by cooling at 20~25℃, centrifuge and dry to obtain industrial magnesium sulfate finished product. The industrial magnesium sulfate is immersed in distilled water, the pH value is adjusted with sulfuric acid, centrifuged, dried and dehydrated, and recrystallized to obtain the magnesium sulfate for medicine.
Application
The ionic strength
The total dissolved solids (TDS) is a parameter considered for different agencies and organizations. The TDS is typically determined by the ionic strength (I). The magnesium sulfate heptahydrate is an electrolyte that produces SO42- and CaCO3 in water and changes its ionic strength. The elimination of therapeutic agents, beverages and other domestic products not used at home, which contain magnesium sulfate heptahydrate, and the agricultural use of fertilizers are sources of magnesium sulfate heptahydrate in the environment. In this work, the complex relative permittivity of liquid samples with different ionic strength levels due to magnesium sulfate heptahydrate dissolved in water is presented. The study covers an ionic strength range from 0 M to 10.553 M. The measurements are performed with an open coaxial probe in a range from 500 MHz to 20 GHz and the Havriliak-Negami model parameters are presented for the different samples. The dielectric constant does not exhibit as high change as loss factor in relation to ionic strength. The loss factor at 500 MHz is used to determine the ionic strength due to magnesium sulfate heptahydrate. The fitting curve shows a second-grade polynomial whose ionic strength goes from 0.0008 M to 8.1179 M and the loss factor at 500 MHz goes from 2.4 to 213.82. Therefore, the open coaxial probe can be used for measuring a wide ionic strength range [1].
As a catalyst
Convenient and simple procedures for the synthesis of phenazine and quinoxaline derivatives were developed via a reaction of o-phenylenediamines and 1,2-dicarbonyl compounds. In addition, the synthesis of two new 1,4-benzodiazine derivatives and the catalytic activity of magnesium sulfate heptahydrate (MgSO4·7H2O) in the room temperature condensation of o-phenylenediamines and 1,2-dicarbonyl compounds in ethanol as solvent are reported. This method has many appealing attributes, such as excellent yields, short reaction times, and simple work-up procedures [2].
Recovery of magnesium sulfate heptahydrate
Eutectic freezing, characterized by a simultaneous crystallization of ice and salt, was applied to recover magnesium sulphate heptahydrate from a magnesium sulphate industrial stream emitted from flue gas desulphurization. Key aspects of the recovery process for further pilot-scale investigation were studied in a laboratory-scale batch crystallizer with focus on discovering the type of the hydrate product and the quality of the ice and salt crystals. MgSO4·12H2O was formed in the crystallizer and the recrystallization into magnesium sulphate heptahydrate (MgSO4·7H2O) occurred spontaneously during the subsequent filtration and drying above 0.4 degrees C. The ice and salt crystal products were reasonably pure (only a small amount of Mn was built into the heptahydrate crystals). Ice crystals were disk-shaped with an average size of 300 mu m. The MgSO4·7H2O crystals were rather needle-like with an average size of 275 mu m. Both products were easily filtered. The characteristics of the eutectic freezing process, i.e. the working area and the dynamics of the simultaneous crystallization, are demonstrated experimentally [3].
Preparation of ZnO and MgZnO nanoparticles
ZnO and MgZnO nanoparticles were prepared by the co-precipitation method utilizing zinc sulfate heptahydrate and magnesium sulfate heptahydrate; structural measurements were also carried out. An x-ray diffraction (XRD) study indicated that no peaks for other possible phases such as MgO or MgZn intermetallic compounds indicating pure wurtzite structure. All nanoparticles crystallized in a hexagonal wurtzite structure with different orientation diffraction peaks; the main peaks were (100), (002), and (101). Grain size (D) increased with increasing Mg concentrations. A scanning electron microscopy (SEM) analysis revealed that nanoparticle size increased by increasing the Mg concentration in a good qualitative with Scherrer equation and not only the size even the grain shape changed. In addition, optical measurements were taken infer that the band gap energy (Eg), extracted from Tauc's plot, decreases with increasing of the Mg concentration doped, and found to be between 3.255 eV and 3.169 eV. The photoluminescence (PL) emission spectra show two peaks at the ultraviolet and green regions [4].
[1] Hernandez-Gomez E S, Olvera-Cervantes J L, Corona-Vasquez B, et al. Open-Ended Coaxial Probe Technique for the measurement of the ionic strength due to magnesium sulfate heptahydrate in water[C]//2021 18th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE). IEEE, 2021: 1-6.
[2] Karami B, Khodabakhshi S. A facile synthesis of phenazine and quinoxaline (new 1, 4-benzo diazine) derivatives using magnesium sulfate heptahydrate as a catalyst[J]. Journal of the Serbian Chemical Society, 2011, 76(9): 1191-1198.
[3] Himawan C, Kramer H J M, Witkamp G J. Study on the recovery of purified MgSO4· 7H2O crystals from industrial solution by eutectic freezing[J]. Separation and purification technology, 2006, 50(2): 240-248.
[4] Hussain H, Albrithen H A, Alshammari A, et al. Investigation of magnesium addition in ZnO matrix using group II heptahydrate[J]. Materials Research Express, 2021, 8(4): 045011.
Related articles And Qustion
See also
Lastest Price from Magnesium sulfate heptahydrate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 10034-99-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $30.00-3.00/kg2025-04-21
- CAS:
- 10034-99-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20MT