Introduction and Pharmacological action of Chrysin
General description
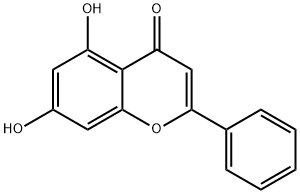
Chrysin, with the CAS No: 480-40-0, also known as chrysanthin, is a yellow powder with the chemical name 5,7-dihydroxy-2-phenyl-4H-chromen-4-one. It is a kind of flavonoid compound extracted from the violet plant wood butterfly, and it is also the main active ingredient of propolis. The chemical structural formula of chrysin is shown in Figure 1. Its chemical molecular formula is C15H10O4, and its molecular weight is 254.24. It is soluble in common organic solvents and insoluble in water. Chrysin is a dihydroxyflavone in which the two hydroxy groups are located at positions 5 and 7. It has a role as an anti-inflammatory agent, an antineoplastic agent, an antioxidant, a hepatoprotective agent, an EC 2.7.11.18 (myosin-light-chain kinase) inhibitor and a plant metabolite. It is a dihydroxyflavone and a 7-hydroxyflavonol, which widely exists in many plants, propolis and honey [1].
Figure 1 The molecular formula of Chrysin
Structure- activity [2-3]
Chrysin is one of the most important bioactive components in different fruits, vegetables and even mushrooms because of its ubiquitous 15-carbon flavone skeleton. Chrysin consists of two fused rings A and C and a benzene ring B, attached to the second position of the C ring. It has a structure common to flavonoids with additional hydroxyl groups at positions 5 and 7 of the A ring (Figure 1). Structurally, chrysin consists of two benzene rings and one heterocyclic ring. Biologically, chrysin exerts many different physiological activities. The antioxidant activity of chrysin is attributed to the presence of a double bond between the carbonyl groups on the C2-C3 and C4 atoms. Unlike many flavonoids that have -OH groups on the C3 and C4 atoms of the B ring, chrysin lacks oxygenation in the B and C rings, a structural property that determines the primary biological activity of chrysin. In addition, the presence of -OH groups on the C5 and C7 atoms of the chrysin structure is related to the free radical scavenging activity.
Pharmacological action
Anti-inflammatory and antioxidant effects [4-5]
A large number of studies have shown that chrysin has anti-inflammatory and antioxidant properties. The anti-inflammatory activity of chrysin is mainly manifested in that it can reduce the activity of transcriptional regulators of inflammatory factors and reduce the production of pro-inflammatory cytokines and inflammatory mediators. Studies have shown that chrysin inhibits monocyte secretion of IL-1β, IL-2, IL-6, IL-12, and TNF-α, promotes IL-4 secretion, and reduces metabolically active succinate in LPS-stimulated PBMCs Dehydrogenase activity, while down-regulating the expression of iNOS, COX-2 and NF-kB (Figure 2). Chrysin can also exert anti-inflammatory effects by activating the activity of peroxisome proliferator activated receptor gamma (PPARγ).
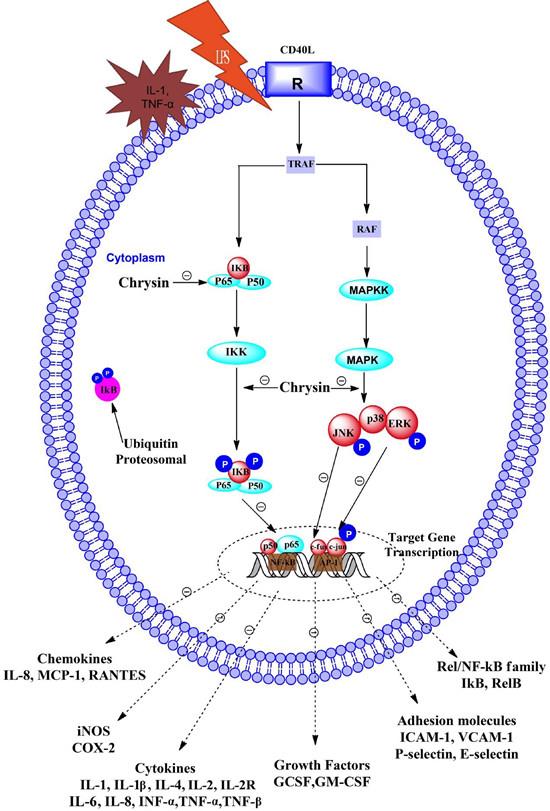
Figure 2 Mechanism of pharmacological action of Chrysin
Oxidative stress is the result of an imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense system, and is a pathological stress state that interferes with normal life activities. In the study, it was found that chrysin can reduce the generation of reactive oxygen species and increase the activity of superoxide dismutase (SOD), thereby reducing the oxidative stress damage of human venous endothelial cells and improving the antioxidant capacity of cells. The antioxidant activity of chrysin is because it has the basic structure of the antioxidant activity of flavonoids, that is, it has two hydroxyl groups and one carbonyl group, and the hydroxyl groups provide hydrogen atoms to free radicals, so chrysin with this structural feature has strong Restorative. In addition, chrysin can inhibit the activity of monoamine oxidase (MAO) by scavenging free radicals, and exert an antioxidant effect.
Antiviral
Chrysin has anti-human immunodeficiency virus (Human immunodeficiency virus, HIV) effect. Studies have shown that chrysin plays an anti-HIV role by inhibiting the fusion of HIV-infected cells, inhibiting the infection and replication of the virus. Enterovirus 71 (EV71) is the most important type of virus that causes hand, foot and mouth disease in children, which can cause serious physical complications and cause great harm [6]. Studies have found that chrysin can inhibit the protease activity encoded by EV71, thereby significantly inhibiting the replication of viral RNA, but without obvious cytotoxicity. Stronger antiviral activity.
Antihypertensive
Various studies have shown that chrysin has antihypertensive effects. Some researchers induced rats into a rat model of pulmonary hypertension and observed the effect of chrysin on cardiovascular remodeling. The results showed that after the intervention of chrysin, it significantly inhibited pulmonary arterial hypertrophy and the expression of collagen type I and III in hypoxic pulmonary hypertension rats, inhibited the proliferation of pulmonary artery smooth muscle cells induced by hypoxia, down-regulated the expression of collagen type I and III, and down-regulated pulmonary artery NOX4. expression. These results suggest that chrysin has antihypertensive effects and can be used as a potential drug for the treatment of cardiovascular disease [7].
Promote wound healing
Studies have shown that nitric oxide (NO) can participate in all processes including extracellular matrix degradation, endothelial cell proliferation, migration and formation of network structures, and lumen formation angiogenesis in the manner of primitive blood vessel formation, and is an important mediator of angiogenesis. Therefore, chrysin linked to NO donor is considered by scholars to promote angiogenesis and provide nutrients for wound healing. In studies, it was found that chrysin can promote the expression of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and cluster of differentiation 31 (CD31), thereby promoting the generation of fibroblasts and pro-angiogenic endothelial cells [8]. Therefore, chrysin is considered as a potential TCM monomeric wound healing agent, which can be applied in regenerative medicine.
Antitumor [9]
In addition to the above pharmacological effects of chrysin, research reports on its anti-tumor have gradually increased in recent years. Studies have shown that chrysin can induce apoptosis in cervical cancer, breast cancer, prostate cancer, and liver cancer. Its anti-tumor mechanism is relatively complex. Chrysin can directly inhibit the growth and proliferation of tumor cells, and can also inhibit the growth and proliferation of tumor cells through other ways. The mechanism may be that chrysin inhibits PI3K/AKT signaling pathway and mitochondrial dysfunction. A variety of studies have shown that chrysin can also assist anti-tumor drugs to improve the effect of inducing tumor cell apoptosis.
Preparation
Natural plant extraction
The main sources of chrysin are honey and propolis, of which the content of chrysin in honey is 5.3 mg/kg and that in propolis is 28 g/L. In addition, chrysin is also found in various vegetables, fruits, herbs, and even mushrooms [10]. Another source of populin is an endophytic fungus Chaetomium globosum, which is related to Chaetomorpha media from India. In addition, chrysin exists in the form of glycosides in walnut, walnut skin, walnut flower and pericarp passion fruit, and in the form of 6-c-arabinoside-8-c-glucoside or glucuronic acid ester in Pinellia ternata.
Chemical synthesis [11]
At present, chrysin is mainly obtained from plant raw materials. However, due to the limitation of natural plant resources and the low content of chrysin, it is limited to obtain chrysin in large quantities by this method. Therefore, chemical synthesis is another promising way to obtain a large amount of chrysin (Figure 3).
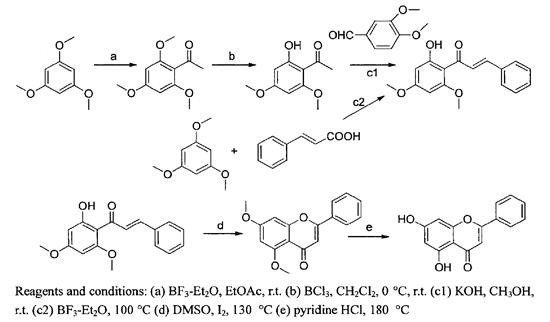
Figure 3 The synthetic route of chrysin
In the first method, 1,3,5-trimethoxybenzene was used as raw material to obtain chrysin through Friedel crafts acetylation, selective demethylation, clarisson Schmidt condensation, intramolecular cyclization and total demethylation.
The second method mainly uses 1,3,5-trimethoxybenzene and cinnamic acid to carry out acylation reaction and selective demethylation reaction, which is completed by one pot cooking. Compared with the first method, it avoids the use of clarisson Schmidt condensation reaction and greatly shortens the reaction time.
References
1. National Center for Biotechnology Information. "PubChem Compound Summary for CID 5281607, Chrysin" PubChem.
2. LI Y, LI Y P, HE J, et al. The Relationship between Pharmacological Properties and Structure-Activity of Chrysin Derivatives[J]. Mini Rev Med Chem, 2019, 19(7): 555-68.
3. TALEBI M, TALEBI M, FARKHONDEH T, et al. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin[J]. Cancer Cell Int, 2021, 21(1): 214.
4. J X, H Z, Y Y, et al. Chrysin attenuates experimental autoimmune neuritis by suppressing immuno-inflammatory responses[J]. Neuroscience, 2014,262:156-164.
5. Zeinalia M, Rezaeec S A, Hosseinzadeha H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances[J]. Biomedicine & Pharmacotherapy, 2017,92:998-1009.
6. Jianmin W, Ting Z, Jiang D, et al. Anti-Enterovirus 71 Effects of Chrysin and Its Phosphate Ester[J]. Plos One, 2014, 9(3): e89668.
7. Li X, Wang X, Li S. Effects of chrysin (5,7-dihydroxyflavone) on vascular remodeling in hypoxia-induced pulmonary hypertension in rats[J]. Chin Med, 2015,10(4).
8. George M Y, Ahmed E, Tadros G M, et al. In vivo cellular and molecular gastroprotective mechanisms of chrysin; emphasis on oxidative stress, inflammation and angiogenesis[J]. European Journal of Pharmacology, 2018,818:486-498.
9. Li X, H J, W J, et al. Combination of chrysin and cisplatin promotes the apoptosis of HepG2 cells by up-regulating p53[J]. Chem Biol Interact, 2015, 232: 12-20.
10. NAZ S, IMRAN M, RAUF A, et al. Chrysin: Pharmacological and therapeutic properties[J]. Life Sci, 2019, 235(116797.
11. Zhang Ji. Synthesis studies of natural flavonoids[D]. Kunming University of Technology, 2014.
Related articles And Qustion
See also
Lastest Price from Chrysin manufacturers

US $1.00-4.00/KG2025-09-08
- CAS:
- 480-40-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $0.00/kg2025-04-29
- CAS:
- 480-40-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg