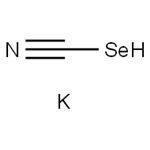
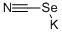Potassium selenocyanate: a useful selenium source
Introduction
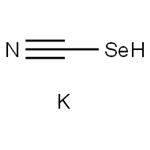
Potassium selenocyanate (KSeCN) is an easy-to-handle and readily available reagent. It is crystalline, colourless, highly hygroscopic, and air-sensitive. Crystalline Potassium selenocyanate is hygroscopic and decomposes with the elimination of selenium upon prolonged exposure to air. Therefore, it should be stored in sealed containers. This salt is soluble in protic and non-protic polar solvents such as DMF, DMSO, NMP, and acetonitrile. This reagent is commercially available and seldom preparedprepared in the laboratory. It can be synthesized from metallic selenium and potassium cyanide in hot water or ethanol. Potassium selenocyanate is a malodorous and highly toxic compound.
Uses
Methodologies employing KSeCN as the selenium source in synthesising organic selenocyanates and isoselenocyanates are practical and versatile[1]. Many organoselenium compounds can be prepared by using Potassium Selenocyanate (KSeCN) such as organoselenonitrile and diorgano diselane which is easily prepared by the alkaline hydrolysis of organoselenonitrile. Because this functional group has a moderate reactivity, its conversion into other functional groups is highly interesting. It allows the formation of new C–Se bonds and the further generation of compounds with significant synthetic, pharmacological, and biological value.
Reactions
(A) KSeCN is an effective source of selenium in synthesising symmetrical diaryl selenides 2[2]. For example, Nageswar and co-workers have developed a methodology for synthesising 2 with aryl halides 1 catalyzed by recyclable CuO nanoparticles under ligand-free conditions in DMSO, using KOH as the base (conditions a). Rao and co-workers reported synthesising 2 by copper-catalyzed cascade reactions with 1 and CuI–trans-1,2-diaminocyclohexane (L1) complex in water and using Cs2CO3 as the base (conditions b). Under both conditions, various aryl halides reacted with KSeCN to give the corresponding products a high yield.
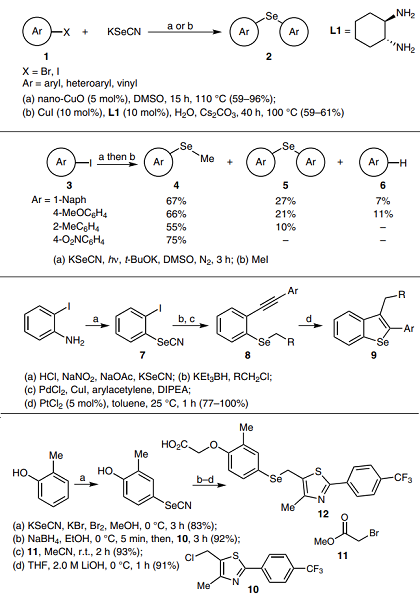
(B) Bouchet, Peñéñory and Argüello synthesized aryl methyl selenides 4 and diaryl selenides 5 employing KSeCN and aryl iodides 3 using base-assisted photoinduced electron-transfer reactions. Aryl selenolate anions can be formed in the presence of t-BuOK as an entrainment reagent. Then, it can react with MeI or 3, yielding 4 or 5, respectively. The authors undertook a comparative study of a set of selenium sources in this work.
(C) It is well known that the selenocyanate anion can be introduced into arenes by diazonium salt formation, followed by nucleophilic displacement. Nakamura and co-workers used this methodology to obtain 1-iodo-2-selenocyanatebenzene (7) as an intermediate to generate alkyl ortho-alkynylphenyl selenides 8, which cyclize in the presence of platinum, rendering 2,3-disubstituted benzo[b]selenophenes 9. That cyclization proceeds by carboselenation by adding a C–Se bond to the alkyne, followed by a direct 1,3-migration of the CH2R group.
(D) In the presence of an oxidizer, such as Br2, KSeCN forms (SeCN)2, which plays the role of the electrophile in electrophilic aromatic substitution reactions. This methodology was used by Sharma et al. for the synthesis of thiazole 12. This compound is a powerful PPARβ/δ ligand, which may possess anti-cancer properties.
References:
[1] HEREDIA A. Potassium Selenocyanate[J]. Synlett, 2014, 14 1. DOI:10.1055/s-0033-1340638.You may like
See also
Lastest Price from Potassium selenocyanate manufacturers
US $1.50/g2025-09-25
- CAS:
- 3425-46-5
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 100 Tons
US $49.00/KG2025-09-05
- CAS:
- 3425-46-5
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000 tons