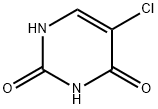
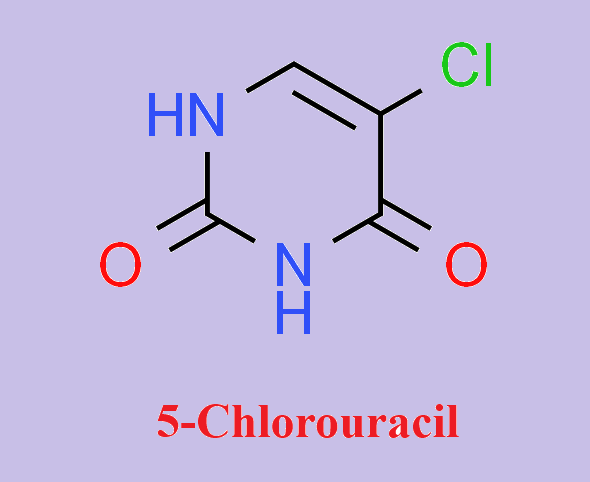
5-Chlorouracil: Uses, Functions and Safety
5-Chlorouracil (5-ClU) is a thymine antagonist with some anti-tumor properties. It is considered a potent mutagen, clastogen and poison, and a potent inducer of sister chromatid exchange. 5-Chloro-2′-deoxyuracil (ClU) can be used to study the structure, stability and function of DNA duplexes in place of T. In addition, inflammation-mediated hypochlorous acid (HOCl) can damage DNA, DNA precursors and other biomolecules, resulting in a range of damage products such as 5-ClU. The formation, binding and repair of 5-ClU can promote transition mutations and other forms of heritable DNA damage.
Functions
Photolysis Effects
5-Chlorouracil is a potentially hazardous substance formed during natural water chlorination and undergoes photolysis when exposed to natural ultraviolet wavelengths in solution. The undissociated form of 5-ClU does not degrade in solution, even when exposed to ultraviolet light of wavelengths shorter than those that penetrate the Earth's atmosphere, but the monoanion, which is dominant above pH 8, disappears at a first-order rate when exposed to light at 290-320 nm. Changes in pH and temperature have a significant effect on the rate of photolysis, with higher pH and temperature resulting in faster decomposition. Under certain conditions of pH, temperature, transparency, depth, and light, the photolysis half-life of 5-ClU may be as low as two days, but in most cases degradation is much slower.
Antitumor Effects
5-Chlorouracil and 5-chlorouracil-histidine can form complexes with certain metal(III) ions such as Cr(III), Fe(III), or Al(III). These complexes are polymeric in nature with high decomposition points and are insoluble in water and common organic solvents. 5-ClU coordinates to metal ions via the O atom of C(4)[dbnd]O and the N atom of N(1), while histidine coordinates to metal ions via the O atom of -COO- and the N atom of the -NH2 group. In vitro and in vivo antitumor activity results showed that the chromium(III) complex had significant activity against P815 mouse mastocytoma, but the aluminum(III) complex was less active.
Electron-induced Chemistry of 5-ClU
The anions of the pyrimidine bases are reaction intermediates that lead to radiation-induced genetic damage and play a crucial role in the charge transfer phenomenon of DNA. Substitution of U or T with 5-halouracil derivatives greatly increases the damage, and therefore these compounds are considered sensitizers in radiotherapy. Dissociative electron attachment of 5-ClU cannot be performed directly because the lifetime of the dissociated σ* state is too short and self-dissociation occurs rapidly. Only when the initially formed non-dissociated π* state and σ* state intersect at the extended C-Cl distance can the chlorouracil anion radical be effectively fragmented.
Safety
Due to sewage chlorination, our water environment contains chlorinated organics such as 5-ClU and 5-chlorouridine. Mice that ingest 5-ClU in drinking water will incorporate the base into liver and testicular DNA in large quantities. Another related metabolite, 5-chlorodeoxyuridine, is 5 times more potent than 5-bromodeoxyuridine in inducing sister chromatid exchange in Chinese hamster ovary cells.
References:
[1] SEVIM AKYUZ Tanil A. Investigation of adsorption of 5-Chlorouracil onto montmorillonite: An IR and Raman spectroscopic study[J]. Applied Clay Science, 2018, 164: 1-68. DOI:10.1016/j.clay.2017.08.018.[2] CHERINE H. KIM. Polymerase Incorporation and Miscoding Properties of 5-Chlorouracil[J]. Chemical Research in Toxicology, 2010, 23 4: 705-846. DOI:10.1021/tx900302j.
[3] G.R. SOUTHWORTH C. W G. Photolysis of 5-chlorouracil in natural waters[J]. Water Research, 1976, 10 11: 935-1046. DOI:10.1016/0043-1354(76)90074-9.
[4] K. NARANG D B V Singh. SYNTHESIS, CHARACTERIZATION AND ANTITUMOUR ACTIVITY OF 5-CHLOROURACIL AND 5-CHLOROURACIL-HISTIDINE COMPLEXES WITH SOME METAL(III) IONS[J]. Synthesis and Reactivity in Inorganic and Metal-organic Chemistry, 1998, 145 1: 1831-1848. DOI:10.1080/00945719809351652.
[5] DR. T S. Electron-induced Chemistry of 5-Chlorouracil[J]. Chemphyschem, 2001, 2 11: 635-704. DOI:10.1002/1439-7641(20011119)2:11<677::AID-CPHC677>3.0.CO;2-C.
You may like
See also
Lastest Price from 5-Chlorouracil manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1820-81-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $15.00-10.00/KG2021-07-02
- CAS:
- 1820-81-1
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton