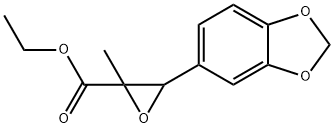
PMK ethyl glycidate: Synthesis and Application
General description
PMK ethyl glycidate can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and pharmaceutical chemical production process.
Synthetic routes
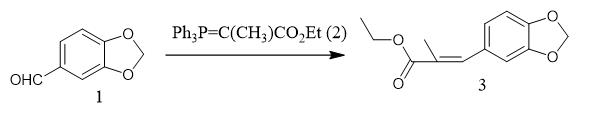
Fig. 1 The synthetic step 1 of PMK ethyl glycidate.
To a solution of piperonal (1, 3.00 g, 19.9 mmol) in CH2Cl2 (100 mL) was added (carbethoxyethylidene)triphenylphosphorane (2,14.5 g, 40.0 mmol), and the reaction mixture was stirred for 24 h at room temperature. The mixture was then concentrated at reduced pressure, and the residue was purified by flash column chromatography on silica gel (hexanes/EtOAc,10:1) to afford olefin 3 (4.45 g, 95%) as a colorless oil: 1H NMR (400 MHz, CDCl3) d 7.58 (s, 1H), 6.92 (d, J = 1.7 Hz, 1H), 6.90 (dd, J = 7.9, 1.7 Hz, 1H), 6.82 (d, J = 8.0 Hz, 1H), 5.97 (s, 2H), 4.24 (q, J = 7.1 Hz, 2H), 2.10 (d, J = 1.6 Hz, 3H),1.33 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) d 168.7,147.6, 138.3, 129.9, 126.9, 124.6, 109.5, 108.2, 101.2, 60.7, 14.3,1 4.0; IR (ATR, cm-1): 2981, 2902,1698, 1628, 1502, 1489,1442, 1258, 1224; HRMS (EI) m/z calcd for C13H14O4 (M+) 234.0892, found 234.0895 [1].
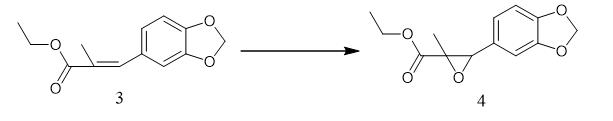
Fig. 2 The synthetic step 2 of PMK ethyl glycidate.
To a solution of olefin 3 (3.00 g, 12.8 mmol) in CH2Cl2 (80 mL) was added 50–55% m-chloroperbenzoic acid (6.2 g,17.9 mmol). The reaction mixture was refluxed for 24 h, cooled to room temperature, quenched with 10% aqueous sodium sulfite (30 mL), and diluted with CH2Cl2 (100 mL). The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 30 mL). The combined organic layers were washed with saturated aqueous NaHCO3 and brine, dried over anhydrous MgSO4, and concentrated at reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (hexanes/EtOAc, 10:1) to afford epoxide 4 (2.12 g, 66%) as a colorless oil: 1H NMR (400 MHz, CDCl3) d 6.79 (d, J = 1.6 Hz, 2H), 6.76 (s, 1H), 5.96 (d, J = 1.6 Hz, 2H), 4.30–4.17 (m, 2H), 4.21 (s, 1H), 1.31 (ddd, J = 8.8, 8.8, 1.7 Hz, 6H); 13C NMR (100 MHz, CDCl3) d 170.8, 147.7, 147.6, 127.6, 120.3, 108.2, 107.0, 101.2, 62.2, 61.8, 59.9, 14.1, 12.5; IR (ATR, cm-1) 2982, 2903, 1726, 1504, 1492, 1444, 1281, 1240; HRMS (EI) m/z calcd for C13H14O5 (M+) 250.0841, found 250.0837 [1].
Application
As an intermediate in organic synthesis
PMK ethyl glycidate as an intermediate in the synthesis of M-ALPHA analog and MDMA. The widespread abuse of illicit psychoactive substances is one of the most serious public health and social problems. A suspicious airmail package was seized by Korean customs, and two psychoactive substances in the grayish-green pills in the package were detected by ultra-performance liq. chromatog. The structures of the two substances were elucidated by a combination of liq. chromatog. quadrupole time-of-flight mass spectrometry, NMR spectroscopy, and comparison with reported or newly generated spectral data of the suggested structures. One of the psychoactive substances proved to be MDMA (commonly known as "Ecstasy"), and the other compd. was an M-ALPHA analog bearing a hydroxyl group and an N-methylcarboxamide group. The new M-ALPHA analog was detd. as 3-(benzo[d][1,3]dioxol-5-yl)-2-hydroxy-N,2-dimethyl-3-(methylamino)propanamide and named as M-ALPHA-HMCA, wherein HMCA denotes hydroxymethylcarboxamide. Although psychoactivity of this compd. has not been assessed, M-ALPHA-HMCA should be considered a potential new psychoactive substance and/or a byproduct of MDMA [1].
PMK ethyl glycidate as an intermediate in the synthesis of desoxy phenethylamine analogs. The desoxy phenethylamine analogs in this study represent a combination of alkyl side-chain and cyclic amines (azetidine, pyrrolidine, piperidine and azepane) to yield a set of mols. of identical elemental compn. as well as major mass spectral fragment ions (base peaks) of identical elemental compn. These desoxy phenethylamine analogs of the aminoketone designer drug, 3,4-methylenedioxy-pyrrovalerone (MDPV) related to the natural product cathinone were prepd. from piperonal (3,4-methylenedioxybenzaldehyde) via the intermediate precursor ketones. The aminoketones and the desoxy phenethylamine regioisomers were each sepd. in capillary gas chromatog. expts. using an Rxi-7Sil MS stationary phase with the aminoketones showing greater retention than the corresponding desoxyamines[2].
References
[1] Lee J H, Park O R, Mandava S, et al. Identification of a new M-ALPHA analog and MDMA in an illegal health product[J]. Forensic science international, 2020, 313: 110332.
[2] Abiedalla Y, DeRuiter J, Clark C R. GC–MS, GC–MS/MS and GC-IR differentiation of desoxy cathinone derivatives: cyclic tertiary amines related to MDPV[J]. Journal of Chromatography B, 2017, 1048: 38-48.
You may like
See also
Lastest Price from PMK ethyl glycidate manufacturers

US $26.00/kg2025-03-28
- CAS:
- 28578-16-7
- Min. Order:
- 25kg
- Purity:
- 99%
- Supply Ability:
- 50MT

US $15.00-9.00/kg2024-08-20
- CAS:
- 28578-16-7
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 500ton