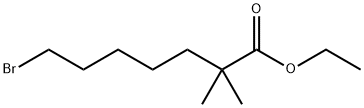
Ethyl-2,2-dimethyl-7-bromoheptanoate:Synthesis and Application
General description
Ethyl-2,2-dimethyl-7-bromoheptanoate is an important raw material in the synthesis route of bempedoic acid. Bempedoic acid (8-Hydroxy-2,2,4,14-tetramethylpentadecanedioic acid), also known as ETC-1002, is a novel lipid-regulating small molecule drug developed by EsperionTherapeutic Company in the United States. As one of the company's primary drug candidates, it targets liver triphosphate lime-lime lyase (ACL) and adenosine monophosphate activated protein kinase (AMPK). Bempedoic acid is a small molecule adenosine-citrate lyase inhibitor that is well tolerated compared to existing statins and can be used in combination with statins to control the elevation of LDL cholesterol..
Synthetic routes
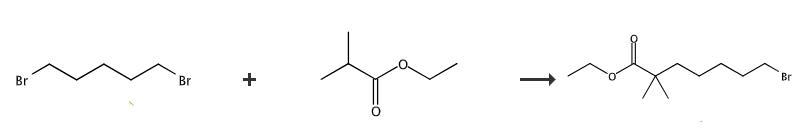
Fig. 1 The synthetic of Ethyl-2,2-dimethyl-7-bromoheptanoate.
Under argon atmosphere, a solution of 1,5-dibromopentane (500 g, 2.2 mol) and ethyl isobutyrate (221 g, 1.9 mol) in anhydrous THF (4 L) was chilled in a dry ice / acetone bath to -78°C. Over a 40 min period, LDA solution in THF (1.8 M, 1 L, 1.8 mol) was added dropwise. After the addition, the solution was allowed to stir overnight and gradually warm to room temperature. Careful quenching of the excess base by slow addition of saturated aqueous NH4Cl solution (3 L) furnished a two-phase mixture. The organic layer was separated and evaporated under vacuum to a minimum volume (ca. 1 L). The organic residue was recombined with the aqueous layer and the resulting mixture was extracted with ethyl acetate (3 x 1 L). The combined ethyl acetate layers were then washed with 1 N HCl (5 L), water (3 L) and saturated aqueous NaHCO3 solution (4 L) before drying over anhydrous MgSO4. Concentration in vacuo gave crude material (468.7 g), which was then purified by distillation affording 205g (208.7 g, 44 %) as a colorless oil. Ethyl 7-Bromo-2,2-dimethylheptanoate (205g), yield 208.7 g, 44 % Bp 106 - 108 °C/0.01 mm Hg. 1H NMR (CDCl3): δ 4.11 (q, 2 H, J = 7.2), 3.39 (t, 2 H, J = 6.8), 1.85 (m, 2 H), 1.56 - 1.35 (m, 4 H), 1.24 (t, 3 H, 7 = 7.2), 1.31 - 1.19 (m, 2 H), 1.16 (s, 6 H). 13C NMR (CDCl3): δ 177.9, 60.2, 42.1, 40.5, 33.8, 32.6, 28.6, 25.2, 24.2, 14.3. HRMS (EI): Calcd for C11H22BrO2 (MH+) 265.0803, found 265.0810 [1].
Application
An important raw material in the synthesis route of bempedoic acid
The present invention provides a synthetic method of a bempedoic acid active pharmaceutical ingredient. The synthetic method comprises: subjecting an organozinc reagent and aldehyde to an addn. reaction in an aprotic solvent to obtain an intermediate, hydrolyzing the intermediate under alk. conditions, and performing acidification to obtain the title compd. Advantageously, the use of the organozinc reagent can greatly reduce reaction byproducts and improve the product yield and purity, and the method has low reaction cost and is suitable for industrial prodn. Bempedoic acid (ETC-1002) is a novel drug for the treatment of elevated LDL cholesterol. Bempedoic acid inhibits the enzyme ATP citrate lyase which is upstream of HMG-CoA reductase, the key enzyme of cholesterol biosynthesis and target of statins [2]. Bempedoic acid is administered as prodrug and activated by a liver-specific enzyme which is not expressed in the skeletal muscles. In randomized trials, a reduction in LDL cholesterol is observed under treatment with bempedoic acid, which is more pronounced in statin-naive patients and synergistic with ezetimibe. Bempedoic acid is also well tolerated by patients with statin-associated muscle symptoms. Bempedoic acid appears to slightly increase uric acid and to improve glucose tolerance. C-reactive protein is reduced by approximately 25%. A cardiovascular outcome trial, enrolling 12600 patients with atherosclerotic cardiovascular disease and statin-associated muscle symptoms is ongoing and expected to report in 2022. Bempedoic acid represents a future therapeutic option for patients not attaining their LDL cholesterol goal especially because of statin-associated muscle symptoms [3].
References
[1] Dasseux J-L, Oniciu CD. Preparation of dicarboxylic acid ketones for cholesterol management and related uses[P]. PCT Int. Appl., 2005068412, 2005.
[2] Ruan J, Yan G, Ruan X et al. Preparation of bempedoic acid[P]. Faming Zhuanli Shenqing, 115108904, 2022.
[3] Katzmann JL, Laufs U. Bempedoic Acid - Mechanism of Action and Clinical Trials[J]. Aktuelle Kardiologie. 2020, 99(04), 381-386.
You may like
Related articles And Qustion
See also

US $0.00-0.00/KG2024-05-20
- CAS:
- 123469-92-1
- Min. Order:
- 1KG
- Purity:
- 97.0-99.0%min GC
- Supply Ability:
- 5-10tons/year
US $8.00-2.00/kg2024-04-24
- CAS:
- 123469-92-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available