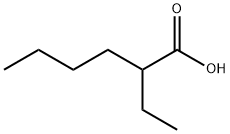
Pharmacology and Biochemistry about 2-Ethylhexanoic acid
2-Ethylhexanoic acid (2-EHA) is a dense, clear, colorless, high boiling liquid and is the most highly produced commercial aliphatic carboxylic acid. It is made via an oxidation of 2-ethyl-1-hexanol or 2-ethylhexanal and is of particular importance in materials science, catalysis, paint industries, and has a variety of commercial uses. It has found widespread use in plasticizers, automotive coolants, synthetic lubricants, metal soaps, for paint driers, PVC stabilizers, wetting agent in emulsions, as a co-solvent, and defoamer in pesticides. Esters of 2-Ethylhexanoic acid, especially those of diglycols, triglycols, and polyethylene glycol are used as plasticizers and stabilizers for PVC polymers. Peroxides of 2-EHA are catalysts in the production of low density polyethylene.

Absorption, Distribution and Excretion
The disposition of di-2-(ethylhexyl)adipate in humans following administration of the stable isotope labeled test substance was investigated. Urine was collected for up to 96 hours after administration. No adverse effects were observed in any of the volunteers and no significant changes in biochemical or hematological parameters were seen. The plasma contained no parent molecule; however, the metabolite 2-ethylhexanoic-acid was detected, but levels were below the limit of quantitation. 2-ethylhexanoic-acid, as a conjugated product, was also the principal metabolite detected in urine. Urinary elimination of the di-2-(ethylhexyl)adipate metabolites peaked within 8 hours of dosing in all volunteers; beyond 36 hours, no metabolites were detected in urine. The conjugated 2-ethylhexanoic acid in urine accounted for an average of 8.6% of the administered dose. A further 3.5% of the dose was accounted for by 2-ethyl-5-hydroxyhexanoic acid, 2-ethylhexanedioic acid, 2-ethyl-5-keto-hexanoic acid, and 2-ethylhexanol. /It was/ concluded that 2-ethylhexanoic acid is an appropriate marker for biological monitoring in estimating the dietary di-2-(ethylhexyl)adipate intake as it is the major metabolite identified and as its rate of elimination is similar to that of other measured di-2-(ethylhexyl)adipate metabolites.[1]
2-(14)C-ethylhexanoic acid in rat blood, brain, liver and kidney was quantitated by liquid scintillation analysis and by wholebody autoradiography in mice. A single intraperitoneal dose of 2-(14)C-ethylhexanoic acid was injected in both species. Animals were sacrificed 30 min, 2 and 6 hr after the administration of 2-(14)C-ethylhexanoic acid in autoradiography experiments. The highest uptake of 2-(14)C-ethylhexanoic acid was observed in the liver, kidney and blood in mice. In contrast, low uptake of 2-(14)C-ethylhexanoic acid was seen in the brain. 2-(14)C-ethylhexanoic acid was well detectable in the olfactory bulb and in the salivary gland. In rats, at 2 hr after administration the highest concentration of 2-(14)C-ethylhexanoic acid occurred in blood (0.3%; of the total dose/g tissue). The radioactivity in the liver (0.2%) and kidney (0.1%) was also relatively high. The concentrations of 2-(14)C-ethylhexanoic acid was low in the brain (0.02%). By 6 hr, the radioactivity had decreased rapidly and was hardly measurable at 24 hr after the administration. The results suggest that 2-ethylhexanoic acid is rapidly cleared from the tissues.
[2-14(C)-Hexyl]2-ethylhexanoic acid in corn oil was administered to female Fischer 344 rats either as a single oral gavage at 100 or 1000 mg/kg, or after 14 days of oral unlabeled 2-ethylhexanoic acid (100 mg/kg only). An aqueous solution of [2-14(C)-hexyl]2-ethylhexanoic acid was applied topically at either 100 or 1000 mg/kg and another group of rats received 2-ethylhexanoic acid by intravenous injection (1 mg/kg). Urine, feces, and blood were collected at various intervals for 96 hr. Approximately 72 to 75 percent of the oral dose was excreted in the urine within 24 hr, and <10 percent was excreted after 24 hr. About 50% of the 14(C) was excreted in the first 8 hr after the 100-mg/kg dose versus 20 percent after the 1000 mg/kg dose. Fecal excretion accounted for 7 to 12 percent of both doses. After intravenous injection, 64 percent of the l4(C) was excreted in the urine and 2 percent in the feces. Repeated dosing with unlabeled 2-ethylhexanoic acid (100 mg/kg) appeared to reduce the urinary elimination of 14(C) slightly to 55 percent in urine, whereas the fecal excretion increased to 15 percent in the first 24 hr. After dermal application, approximately 30 percent of the applied dose was excreted in the urine during the first 24 hr, followed by an additional 8 and 17 percent from 24 to 96 hr for the 100- and 1000-mg/kg doses, respectively. Fecal excretion was 7 percent for both dose levels. Dermal absorption was estimated to be 63 to 70 percent relative to intravenous administration. After dermal application, peak blood levels of 14C occurred about 5.7 hr after application and the absorption half-life was 3.2 hr.[2]
Metabolism / Metabolites
Liver microsomes were extracted from male Han/Wistar rats, male DBA/2N/Kuo mice, and humans undergoing liver surgery. 2-Ethylhexanoic acid metabolism was determined in-vitro by incubating the microsomes in the presence and absence of the cytochrome P450 inhibitors triacetyloleandomycin, metyrapone, quinidine, and SKF-525A. In-vivo, 2-ethylhexanoic acid metabolism was determined by administering 2-ethylhexanoic acid to rats with and without the cytochrome P450 inhibitors, and by assessing metabolite levels in human urine samples taken from 2-ethylhexanoic acid exposed sawmill workers. The main 2-ethylhexanoic acid metabolite produced in all microsomes was 2-ethyl-1,6-hexanedioic acid. Production of this metabolite was prevented by triacetyloleandomycin, metyrapone, quinidine, and SKF-525A. /It was/ concluded that some cytochrome P450s may contribute to 2-ethylhexanoic acid metabolism in the liver, that the same 2-ethylhexanoic acid metabolites are formed in rats and humans, and that 2-ethylhexanoic acid resembles the hepatotoxicant 2-envalproic acid. [3]
The metabolites of 2-ethylhexanoic acid ... were investigated in rat urine. Male Wister rats were give 2-ethylhexanoic acid in drinking water 1600 mg/kg daily for nine weeks, and then urine specimens were collected and analysed. ... In addition to 2-(ethylhexyl)adipate, ten different 2-(ethylhexyl)adipate related metabolites were found in the urine of 2-(ethylhexyl)adipate treated rats. The main metabolite was 2-ethyl-1,6-hexanedioic acid. Urine also contained 2-ethyl-6-hydroxyhexanoic acid and five other hydroxylated metabolites and two lactones, the detailed structures of which have not yet been elucidated. The unsaturated 5,6-dehydro-di-2-(ethylhexyl)adipate was also identified; this is the metabolite corresponding to 2-n-propyl-4-pentenoic acid, the hepatotoxic metabolite of valproic acid. At least part of the 2-(ethylhexyl)adipate is present in urine as a glucuronide conjugate.
Mechanism of Action
The enantiomers of 2-ethylhexanoic acid were separated via preparative HPLC to greater than 99.8% optical purity, and greater than 99% purity according to a gas chromatographic analysis. It was demonstrated that the (R)-enantiomer is a more potent teratogen than the (S)-enantiomer for the induction of exencephaly as well as malformations of other organ systems. Pharmacokinetic analyses for each of the enantiomers were performed in maternal plasma, maternal muscle, and embryo. The pharmacokinetics showed that the peak concentration (Cmax) for both enantiomers in the three compartments was approximately equivalent and was attained within 15 min following the third administration. The area under the concentration versus time curve values for the two enantiomers were approximately 10 higher for the (R)-antipode because of a slightly slower elimination of this compound. There was negligible (or no) racemization of the two enantiomers in the biological samples. The results suggest that teratologic differences in the enantiomers of sodium 2-ethylhexanoate are not due to differences in the concentrations of these antipodes in the embryo, but more likely result from the specific interaction of the enantiomers with chiral molecules in the embryo. [4]
References
[1] Loftus NJ et al; Food and Chemical Toxicol 31 (9): 609-14 (1993)
[2] Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:730
[3] Pennanen S et al; Human and Experim Toxicol 15 (5): 435-42 (1996)
[4] Collins MD et al; Toxicol Appl Pharmacol 112 (2): 257-65 (1992)
See also
Lastest Price from 2-Ethylhexanoic acid manufacturers

US $1.00/KG2025-04-21
- CAS:
- 149-57-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 149-57-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month