Pharmacological action and Preparation of Finasteride
General description
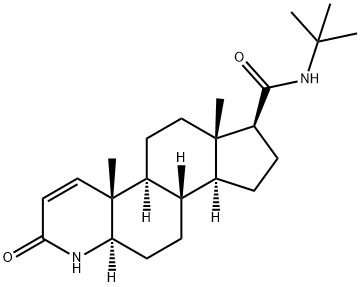
The Finasteride, a 4-azasteroid compound, with the CAS No: 98319-26-7, its chemical name is N-tert-butyl-3-oxo-4-aza-5α-androst-1-ene-17β-amide, which is the result of 15 years of research by Merck & Co. It was first listed in Italy in 1991, put into clinical practice after being approved in the United States in June 1992, and entered China in 1993 [1]. Because it has a good inhibitory effect on 5α-reductase and can significantly reduce the level of dihydrotestosterone (DHT), it is used in the clinical treatment of benign prostatic hyperplasia. This product can reduce the prostate volume in patients with symptomatic prostatic hyperplasia (BPH), increased urine flow and improved symptoms [2]. Finasteride has a molecular formula of C23H36N2O2 and a molecular weight of 372.55 (Figure 1). Its appearance is white or off white crystalline powder. It is odorless. The melting point measured in dry nitrogen atmosphere is 257℃. It is easily soluble in chloroform, methanol, etc., slightly soluble in ether, and insoluble in water.
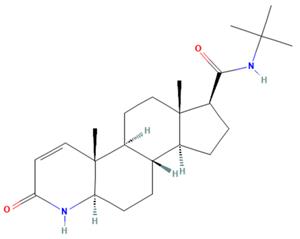
Figure 1. The molecular formula of Finasteride
Pharmacodynamics
Finasteride is a 4-azasteroid that can selectively and competitively inhibits 5α-reductase activity. The conversion of testosterone (T) to dihydrotestosterone (DHT) requires the participation of this nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzyme. Finasteride can specifically inhibit the type II 5α-reductase isoenzyme, which is mainly formed in prostate tissue. Dose-dependent inhibition of 5α-reductase can significantly reduce DHT levels in the prostate (up to more than 90%) and circulating (60% to 80%). Prostate testosterone levels were increased in BPH patients treated with finasteride (approximately 85 %), however, without affecting prostate growth and morphology [3]. This product does not affect the androgen receptor, and has no effect on other hormones such as follicle-stimulating hormone, luteinizing hormone, cortisone, estrogen, prolactin and thyroxine, and does not inhibit the production of adrenal steroids. Serum prostate-specific antigen (PSA) levels were significantly decreased in symptomatic BPH patients treated with finasteride (41 % to 71 %), but the mean ratio of free to total PSA was not affected.
Pharmacokinetics [4]
Absorption: A single oral dose of finasteride (5 mg) has a bioavailability of approximately 63% (34-108%), and its bioavailability is not affected by food. The plasma concentration reaches the peak 1-2 h after taking the drug, and the Cmax is (37ng/ml).
Distribution: Mean steady-state volume of distribution is 76 L (range 44-96 L) and plasma protein binding is approximately 90%. Multi dose oral Logistics Department slowly accumulates a small amount. Taking finasteride (5mg/d) continuously for 17 days, the plasma concentration of subjects in the 45-60-year-old age group after multiple oral doses was 47% higher than that after a single oral dose, and the subjects in the age group over 70 years old were tested. The plasma concentration of the patients was 54% higher than that of a single oral dose, and the mean trough concentration was 6.2ng/ml and 8.1ng/ml in the two age groups, respectively.
Metabolism: Finasteride is primarily metabolized in the liver by the cytochrome P450 enzyme 3A4, and its two major metabolites play only a minor role in the inhibitory activity of finasteride 5α-reductase.
Excretion: The plasma clearance rate of finasteride was 165ml/min, and the average elimination half-life of plasma was 6 h (range 3-16 h). After a single oral dose of 14C finasteride was given to men, 39% of the administered dose was excreted in the form of metabolites from urine, and 57% of the total amount was excreted from feces. The terminal half-life of finasteride is 8 hours (6-15 hours) in persons over 70 years of age, and no pharmacokinetic studies have been conducted in youth or children under 18 years of age.
Pharmacological action [5]
Finasteride is an antiandrogenic compound that works by suppressing the production of serum and intraprostatic dihydrotestosterone (DHT) in men via inhibiting the enzyme responsible for the biosynthesis of DHT. The maximum effect of a rapid reduction in serum DHT concentration is expected to be observed 8 hours following administration of the first dose. In a single man receiving a single oral dose of 5 mg finasteride for up to 4 years, there was a reduction in the serum DHT concentrations by approximately 70% and the median circulating level of testosterone increased by approximately 10-20% within the physiologic range. In a double-blind, placebo-controlled study, finasteride reduced intraprostatic DHT level by 91.4% but finasteride is not expected to decrease the DHT levels to castrate levels since circulating testosterone is also converted to DHT by the type 1 isoenzyme expressed in other tissues. It is expected that DHT levels return to normal within 14 days upon discontinuation of the drug. In a study of male patients with benign prostatic hyperplasia prior to prostatectomy, the treatment with finasteride resulted in an approximate 80% lower DHT content was measured in prostatic tissue removed at surgery compared to placebo. While finasteride reduces the size of the prostate gland by 20%, this may not correlate well with improvement in symptoms. The effects of finasteride are reported to be more pronounced in male patients with enlarged prostates (>25 mL) who are at the greatest risk of disease progression. In phase III clinical studies, oral administration of finasteride in male patients with male pattern hair loss promoted hair growth and prevented further hair loss by 66% and 83% of the subjects, respectively, which lasted during two years' treatment. The incidences of these effects in treatment groups were significantly higher than that of the group receiving a placebo. Following finasteride administration, the levels of DHT in the scalp skin was shown to be reduced by more than 60%, indicating that the DHT found in scalp is derived from both local DHT production and circulating DHT. The effect of finasteride on scalp DHT is likely seen because of its effect on both local follicular DHT levels as well as serum DHT levels.. There is evidence from early clinical observations and controlled studies that finasteride may reduce bleeding of prostatic origin.
Preparation
The synthetic routes of finasteride (1) reported in the literature can be roughly classified into two categories. Route 1 uses pregnenolone (2) as the starting material to synthesize 3-carbonyl 4-androstene-17β-carboxylic acid (6) After that, finasteride is synthesized through multi-step reactions such as ring opening, hydrogenation, amidation, and dehydrogenation (Figure 2).
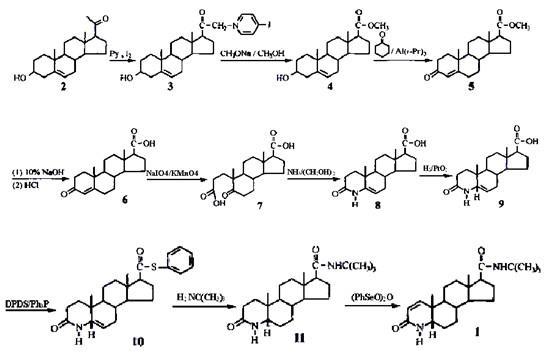
Figure 2. Synthetic route Ⅰ of finasteride
Route II uses pregnenolone acetate (2') as raw material, which is oxidized by sodium hypobromate and methylated by methanol to obtain 3-carbonyl 4-androstene-17β- Methyl formate (5′) is then oxidized by oppenauer, oxidized by double bonds, ammonolysis and cyclization, hydrogenation of double bonds, dehydrogenation at 1,2 positions, and bodroux reaction to prepare finasteride (Figure 3).
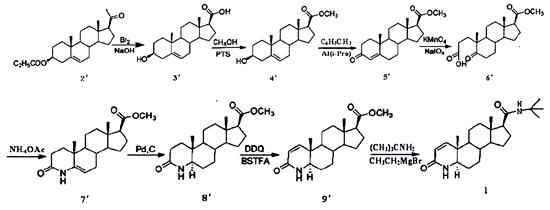
Figure 3. Synthetic route II of finasteride
The disadvantage of route Ⅰ is that the (PhSeO)2O used in the 1,2-position dehydrogenation process is highly toxic, which is not conducive to environmental protection, and ammonia gas is introduced into the ring-closing process of nitrogen hybridization, which requires high reaction equipment [6]. In route II, the reaction product of DDQ and N,N-bis(trimethylsilyl)trifluoroacetamide (BSTFA) is used as the dehydrogenation reagent during dehydrogenation, avoiding highly toxic selenium compounds and reducing environmental pollution, which fundamentally solves the 1,2-bit dehydrogenation problem that is common in the current factory [7].
References
1. Bartsch G, Rittmaster R S, Klocker H. Dihydrotestosterone and the role of 5 alphareductase inhibitors in benign prostatic hyperplasia[J]. Der Urologe, 2002, 41(5):412-424.
2. Peters D H, Sorkin E M. Finasteride: A review of its potential in the treatment of benign prostatic hyperplasia[J]. Drugs, 1993, 46(1):177.
3. Wai Wei. Synsynthesis of finasteride[D].Jilin University, 2006.
4. https ://www.drugbank.ca/drugs/DB01216.
5. National Center for Biotechnology Information. "PubChem Compound Summary for CID 57363, Finasteride" PubChem.
6. Zang Hongxia. Finasteride synthesis roadmap for the treatment of benign prostate hyperplasia[J]. Journal of Xingtai University, 2010,25(04):123-124.
7. Yang Yan, Ma Hongmei, Qu Zhang, Jin Yongsheng, Hu Honggang.Synthesis of finasteride[J]. Journal of Pharmaceutical Practice, 2007(05):310-312.
You may like
Related articles And Qustion
See also
Lastest Price from Finasteride manufacturers
US $1.00/KG2025-10-14
- CAS:
- 98319-26-7
- Min. Order:
- 10000KG
- Purity:
- 99.99%
- Supply Ability:
- 20 TONS

US $0.00/box2025-09-24
- CAS:
- 98319-26-7
- Min. Order:
- 1box
- Purity:
- 0.99
- Supply Ability:
- 10tons