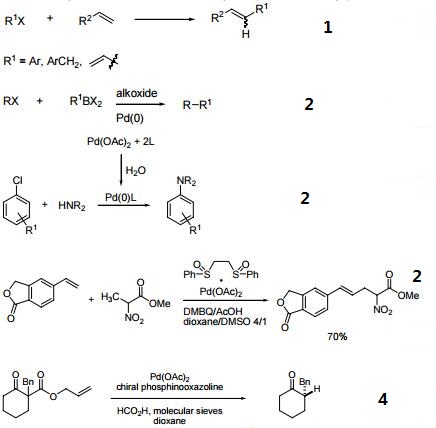
Pd(OAc)2/Xantphos Catalyst System
Org. Process Res. Dev.2020, 24, 228. DOI: 10.1021/acs.oprd.9b00495
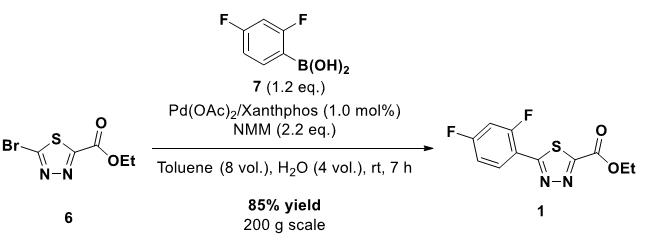
◆A recent report by Eastgate and Blackmond on a Pd/Xantphos-catalyzed C−H arylation reaction suggested that under their reaction conditions, the active ligand was not Xantphos or Xantphos bisphosphine oxide, but the bisphosphine monoxide form of Xantphos.
◆They also proved that the addition of excess free Xantphos relative to the Pd-source significantly inhibited the coupling. It was concluded that nonoxidized Xantphos has a higher binding strength to Pd as the bisphosphine monoxide form (and the bisphosphine oxide) and therefore the Pd-center would be saturated with free Xantphos, resulting in the formation of an inactive catalyst.
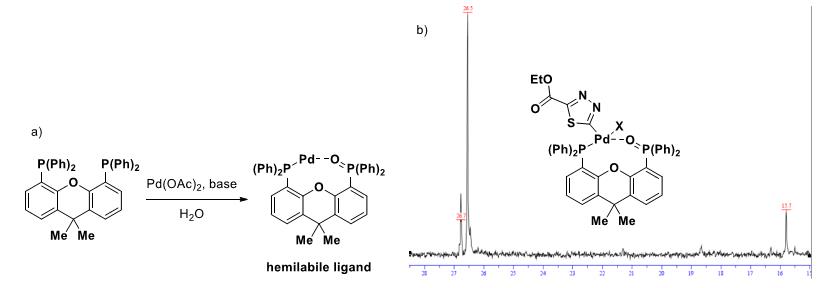
◆We observed the same phenomenon: when a 3-fold excess of Xantphos relative to Pd(OAc)2 was used, the reaction basically shut down and only 7% conversion of bromo thiadiazole 6 was obtained after 2 h atroom temperature (ca. 20% after 18 h).
◆In addition, analyzing an equimolar mixture of 6, Xantphos, and Pd(OAc)2 in d8-toluene/D2O/NMM by 31P NMR showed two phosphorus resonances at 15.7 and 26.7 ppm, which is most likely in accordance with the formation of a highly active Xantphos mono-oxide complex of Pd (X = Br or NMM) in our Suzuki-coupling (signal at 26.5 ppm corresponds to Xantphos bis-oxide).
Note
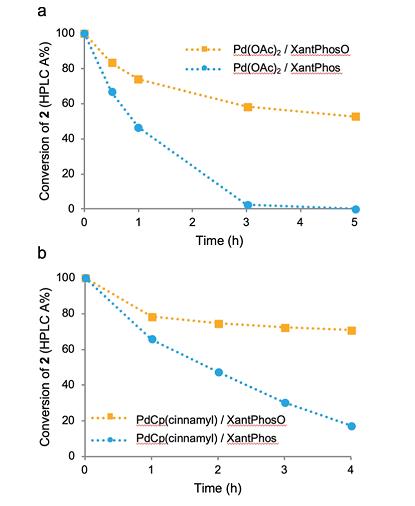
◆In the reaction above, the conversion of bromide 2 using XantPhos was substantially faster, potentially suggesting that, for this reaction system, ligand oxidation is not a path to a more active catalyst but rather a likely mode of catalyst deactivation.
Related articles And Qustion
Lastest Price from Palladium (II) Acetate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 3375-31-3
- Min. Order:
- 1KG
- Purity:
- 47.5%min
- Supply Ability:
- 6tons

US $6.00/kg2025-04-21
- CAS:
- 3375-31-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month