What is Palladium (II) Acetate?
Identification
Product Name: Palladium (II) Acetate
Synonyms: ACETIC ACID PALLADIUM(II) SALT;PALLADIUM ACETATE;PALLADIUM(+2)ACETATE;PALLADIUM DIACETATE;PALLADIUM(II) ACETATE;PALLADIUM(II) ACETATE IN IONIC LIQUID ON SILICA;Pd(OAC)2 (=Palladium Acetate);PALLADIUM (II) ACETATE, TRIMER
CAS: 3375-31-3
MF: C4H6O4Pd
MW: 224.51
EINECS: 222-164-4
Properties
Melting point 205 °C
Storage temp. Store at R.T.
Solubility Soluble as monomer in glacial acetic acid or as trimer in benzene.
Form Various Forms In Red-(Powder/Flake/Crystalline/Beads)
Color Red-brown
PH 2-3 (H2O, 20℃)(aqueous suspension)
PH Range 2.0 - 3.0 at 20 °C
Water Solubility insoluble
Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.
Uses
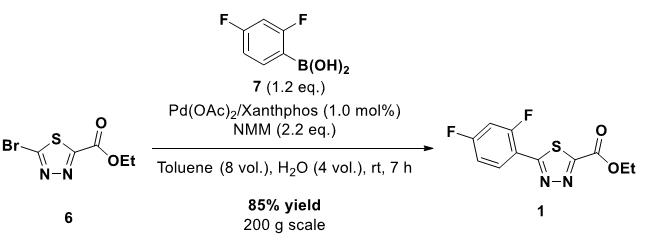
Palladium (II) Acetate Trimer is used in Suzuki-Miyaura cross-coupling reactions. It also serves to catalyze the chemoselective reduction of nitroarenes.
Reactions
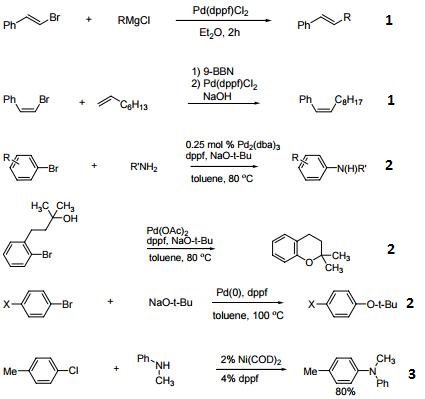
Efficient catalyst for the arylation of olefins (Heck reaction).
Catalyst for cross-coupling reactions.
Catalyst for C-H activation.
Precatalyst for enantioselective decarboxylative protonation of allyl β-ketoesters.
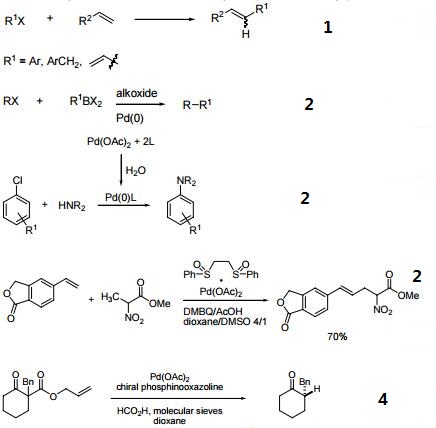
Precursor to other Pd compounds
Palladium acetate is used to produce other palladium(II) compounds. For example, phenylpalladium acetate, used to isomerize allyl alcohols to aldehydes, is prepared by the following reaction:
Hg(C6H5)(OAc) + Pd(OAc)2 → Pd(C6H5)(OAc) + Hg(OAc)2
Palladium(II) acetate reacts with acetylacetone (the "acac" ligand) to produce Pd(acac)2.
Light or heat reduce palladium acetate to give thin layers of palladium and can produce nanowires and colloids.
Catalysis
Palladium acetate is a catalyst for many organic reactions, especially alkenes, dienes, and alkyl, aryl, and vinyl halides to form reactive adducts.
Reactions catalyzed by palladium(II) acetate:
Vinylation: An example is the Heck reaction and related processes.
Rearrangement of acyclic dienes: An example is the Cope rearrangement
Carbonylation reactions: for example, the formation of esters from aryl iodides, carbon monoxide, an alcohol or phenol.
Reductive amination of aldehydes or ketones by potassium formate.
Wacker process: the oxidation of ethylene by water to acetaldehyde (precursor to poly(vinyl acetate).
Buchwald-Hartwig amination of aryl halides/pseudohalides with alkyl an aryl amines.
conversion of aryl bromides into the trimethylsilanes, a functional group in many organic compounds including the fungicide "Latitude".
RC6H4Br + Si2(CH3)6 → RC6H4Si(CH3)3 + Si(CH3)3Br
Pd(O2CCH3)2 is compatible with the electronic properties of aryl bromides, and unlike other methods of synthesis, this method does not require high pressure equipment.
Related articles And Qustion
See also
Lastest Price from Palladium (II) Acetate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 3375-31-3
- Min. Order:
- 1KG
- Purity:
- 47.5%min
- Supply Ability:
- 6tons

US $6.00/kg2025-04-21
- CAS:
- 3375-31-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month