PBr3: A Polar Molecule with Diverse Applications
Phosphorus tribromide, commonly referred to as PBr3, is a colorless liquid with a pungent odor. It is utilized in laboratories for the transformation of alcohols to alkyl bromides. This article explores the polar nature of PBr3, its synthesis, applications, and potential risks associated with its use.
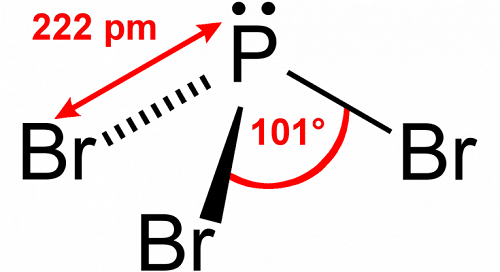
Polar Nature of PBr3
PBr3 is considered a polar molecule due to its asymmetrical shape. The electronegativity difference between bromine (Br) and phosphorus (P) atoms causes the P-Br bonds to be polar. Despite its asymmetry, PBr3 retains a net dipole moment because the individual dipole moments of its bonds do not cancel each other out.
Synthesis of PBr3
PBr3 is prepared by reacting red phosphorus with bromine in the presence of a diluent. The reaction is highly exothermic and is conducted using an excess of phosphorus to prevent the formation of PBr5. The reaction equation is as follows:
2P + 3Br2 → 2PBr3
Applications of PBr3
Alkyl Bromide Formation: PBr3 is commonly used in laboratory settings to convert alcohols to alkyl bromides. This reaction is an essential step in various organic synthesis processes.
Hell-Volhard-Zelinsky Halogenation: PBr3 serves as an intermediate in this halogenation reaction, which involves the substitution of a carboxylic acid with a halogen atom.
Catalyst in Alpha Bromination: PBr3 acts as a catalyst in the alpha bromination of carboxylic acids, enabling the introduction of a bromine atom at the alpha position.
Pharmaceutical Manufacturing: PBr3 finds applications in the synthesis of several pharmaceuticals, including fenoprofen and alprazolam, among others.
Fire Suppressant: PBr3 exhibits properties that make it a potent fire suppressant. Its use in fire suppression systems helps prevent the spread of flames and minimize fire-related hazards.
Risks and Precautions
While PBr3 serves various purposes, it is crucial to handle it with caution. The compound undergoes rapid hydrolysis and can produce toxic compounds like phosphine and HBr3 in certain reactions. Exposure to these substances can be harmful to health. Therefore, appropriate safety measures and protocols should be followed when working with PBr3.
Conclusion
To summarize, PBr3 is a polar molecule due to its asymmetrical shape and the non-cancellation of dipole moments. Its polar nature makes it useful in various chemical reactions, particularly in the conversion of alcohols to alkyl bromides. PBr3 also finds application as a catalyst, in fire suppression systems, and in pharmaceutical manufacturing. However, its use requires careful handling due to potential risks associated with the production of toxic compounds.
You may like
Related articles And Qustion
See also
Lastest Price from Phosphorus tribromide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7789-60-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-03-07
- CAS:
- 7789-60-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons