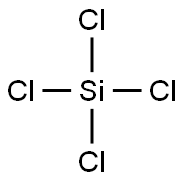
Is SiCl4 Polar or Nonpolar?
Description
In chemistry, silicon tetrachloride (SiCl4; Tetrachlorosilane) is a substance with a low boiling point, higher gasification capacity, and a larger relative density; very toxic and corrosive, and it's easy to form a toxic dense cloud of high concentrations of toxic vapors in a short time, resulting in a severe risk to the environment and human. However, Silicon tetrachloride is the main byproduct of the polysilicon industry[1].
Chemical property
SiCl4 is a colorless, noninflammable, volatile liquid with a pungent, suffocating odor. It fumes in the air and is corrosive to metals and tissues in the presence of moisture. It is not soluble in water and, when combined with water, produces silicon dioxide and hydrochloric acid. Silicon tetrachloride is soluble in benzene, chloroform, ether, and hydrochloric acid[2]. It's most typically used to purify elemental silicon, which is used in microchips and other computer applications.
Polar or non-polar
SiCl4 is a non-polar molecule. It comprises one silicon (Si) atom and four chlorine (Cl) atoms. The silicon is kept at the central position while all four chlorine atoms occupy surrounding positions.
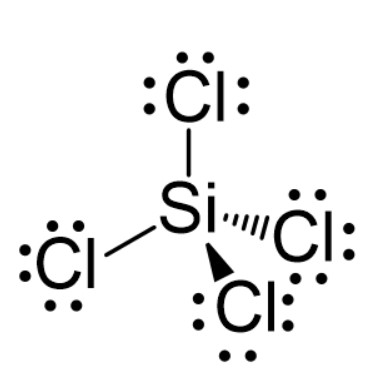
Specifically, the Lewis dot structure of SiCl4 shows four Si-Cl bonds. All four valence electrons of silicon used in covalent bonding denote no lone pair of electrons on the central Si-atom in SiCl4.Contrarily, each terminal Cl-atom contains 3 lone pairs of electrons. Due to the absence of any lone pair of electrons on the central Si-atom in SiCl4, there is no distortion in the symmetrical tetrahedral shape or geometry of the molecule.
The electronegativity difference in SiCl4 between Si-atom (E.N= 1.90) and Cl-atom (E.N= 3.16) in each of the four Si-Cl bonds is 1.26 units. Hence, the chlorine atom with relatively higher electronegativity gains a partial negative (Clδ-) charge, while the silicon atom obtains a partial positive (Siδ+) charge. As a result, in SiCl4, all Si-Cl bonds are individually polar, with an electronegativity difference of 1.26 units. In polar bonds of SiCl4, the dipole moment of each Si-Cl bond points from Siδ+ to Clδ-.
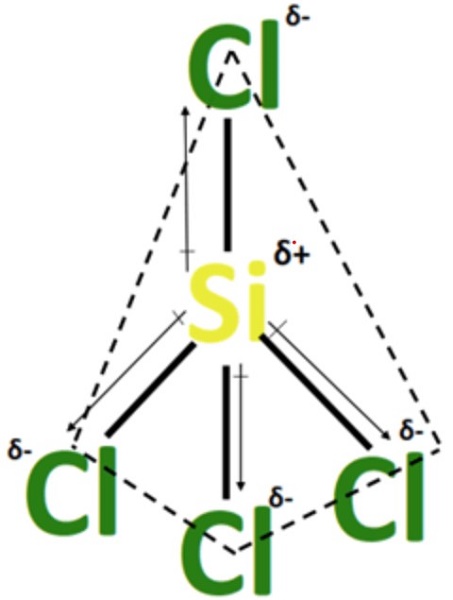
Due to the absence of any lone pair of electrons on the central Si-atom, SiCl4 has an identical molecular and electron geometry that is tetrahedral. Highly electronegative Cl-atoms attract the shared cloud of each Si-Cl to a large extent. Hence, the Si-Cl bonds are individually polar. Still, due to the symmetrical tetrahedral shape of SiCl4, the overall charged electron cloud stays uniformly distributed as the Si-Cl dipole moments get canceled equally in the molecule. The net dipole moment of three downwards-pointing Si-Cl bonds gets balanced with the dipole moment of an upwards-pointing Si-Cl bond. Consequently, silicon tetrachloride (SiCl4) is a non-polar molecule (net µ = 0).
References
[1] Zhang Jianwen . “Numerical investigation on three-dimensional dispersion and conversion behaviors of silicon tetrachloride release in the atmosphere.” Journal of Hazardous Materials 288 (2015): 1-16.
[2] S. López. “Silicon Tetrachloride.”Encyclopedia of Toxicology (Third Edition)(2014): 916-918.
You may like
Related articles And Qustion
See also
Lastest Price from Tetrachlorosilane manufacturers
US $10.00/KG2025-04-21
- CAS:
- 10026-04-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt