Palmitic acid: Sources and Biological Function
Introduction
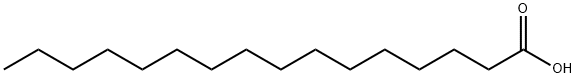
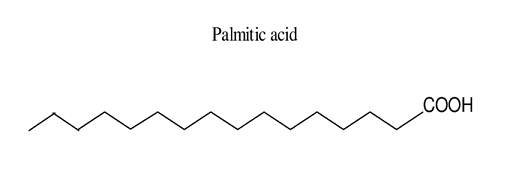
Palmitic acid (16:0, PA) is the most common saturated fatty acid found in the human body. It can be obtained through diet or synthesized endogenously from other fatty acids, carbohydrates, and amino acids. PA constitutes 20–30% of total fatty acids (FA) in membrane phospholipids (PL) and adipose triacylglycerols (TAG). On average, a 70-kg man contains 3.5 kg of PA. The structure of palmitic acid features a 16-carbon backbone. Its molecular formula, C16H32O2, indicates that it contains 16 carbon atoms, 32 hydrogen atoms, and 2 oxygen atoms. Palmitic acid has a molecular weight of 256.42. It is commonly used in personal care products and cosmetics[1].
Sources

As the name suggests, PA is a major component of palm oil (44% of total fats), but significant amounts can also be found in meat and dairy products (50–60% of total fats), cocoa butter (26%), and olive oil (8–20%). Furthermore, PA is present in breast milk, accounting for 20–30% of total fats. The average intake of PA is around 20–30 grams per day, representing about 8–10% of total energy intake. PA tissue content seems to be controlled around a well-defined concentration, as changes in its intake do not significantly influence its tissue concentration due to the counterbalance provided by endogenous biosynthesis via de novo lipogenesis (DNL).
Effect on the Melting Point of Milk Fat
Palmitic acid has been identified as a major contributor to the melting point of milk fat, alongside oleic acid (C18:1 cis9). The melting of milk fat is defined over a range due to the diversity of its composite fatty acid chains. Higher concentrations of palmitic acid have repeatedly been associated with higher melting points in milk fat. Therefore, the milk fatty acid composition and butter texture are influenced by dairy cattle diets, affecting not only the peak melting point but also the onset of melting. Enjalbert et al. (2000) identified that increased palmitic acid content was associated with better butter quality at room temperature, while increased oleic acid content resulted in softness at lower temperatures. Consequently, butter with a lower ratio of palmitic acid to oleic acid (P/O) is expected to be softer[2].
Biological Function
Particular physiopathological conditions and nutritional factors may strongly induce DNL, resulting in increased tissue content of PA and disrupted homeostatic control of its tissue concentration. However, under normal physiological conditions, PA accumulation is prevented by enhanced delta 9 desaturation to palmitoleic acid (16:1n−7, POA) and/or elongation to stearic acid (SA) and further delta 9 desaturation to oleic acid (18:1, OA). The tight homeostatic control of PA tissue concentration is likely related to its fundamental physiological role in several biological functions. Particularly in infants, PA seems to play a crucial role, as recently thoroughly reviewed by Innis. The disruption of PA homeostatic balance, implicated in different physiopathological conditions such as atherosclerosis, neurodegenerative diseases, and cancer, is often related to uncontrolled PA endogenous biosynthesis, irrespective of its dietary contribution.
References:
[1] GIANFRANCA CARTA. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications.[J]. ACS Applied Electronic Materials, 2017. DOI:10.3389/fphys.2017.00902.[2] JANET MUSIC . Data deficits and transparency: What led to Canada’s ‘buttergate’[J]. Trends in Food Science & Technology, 2022, 123: 1-. DOI:10.1016/j.tifs.2022.02.005.
You may like
Related articles And Qustion
See also
Lastest Price from Palmitic acid manufacturers

US $0.00/kg2025-11-21
- CAS:
- 57-10-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $1.00/g2025-08-19
- CAS:
- 57-10-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg