Palladium-Catalyzed Reaction of 2-Hydroxy-2-methylpropiophenone with Aryl Bromides
General Description
The palladium-catalyzed reaction of 2-hydroxy-2-methylpropiophenone involves a unique catalytic sequence of successive multiple arylation through both C-C and C-H bond cleavages. This intriguing reaction pathway expands our understanding of transition metal-catalyzed reactions and provides insights into the activation of inert bonds. Mechanistically, the reaction proceeds through an R-ketol rearrangement, diarylation, reverse R-ketol rearrangement, and triarylation, leading to the formation of tetraarylethanes and isochromanones. The substrate scope of the reaction has been explored using different aryl bromides and ligands, resulting in the formation of diverse products with varying yields. The use of specific ligands and aryl bromides allows for the selective synthesis of specific compounds. Further studies are needed to optimize the reaction conditions and explore the broader substrate scope. Overall, it enhances our knowledge of palladium-catalyzed reaction of 2-hydroxy-2-methylpropiophenone and their potential applications in organic synthesis.

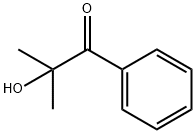
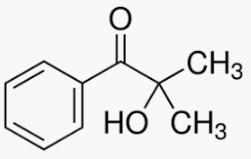
Figure 1. 2-Hydroxy-2-methylpropiophenone
Intriguing Catalytic Sequence of 2-Hydroxy-2-methylpropiophenone
Recently, there has been a growing interest in transition metal-catalyzed organic reactions that involve the cleavage of C-H and C-C bonds. These reactions are attractive from an atom-economic and chemoselective perspective. Researchers have developed various catalytic processes to activate these normally inert bonds. In previous studies, it was reported that phenols, aromatic ketones, and benzamides can undergo regioselective aromatic arylation by cleaving C-H bonds when treated with aryl halides in the presence of palladium catalysts. The success of this reaction depends on the coordination of functional groups to arylpalladium(II) intermediates. Further investigations revealed that the arylation of tert-benzyl alcohols can occur not only through C-H bond cleavage but also via C-C bond cleavage. It was found that the C-C bond cleavage predominates when an appropriate ligand such as PCy3 is used. In a study, a structurally related substrate, 2-hydroxy-2-methylpropiophenone, was subjected to treatment with excess bromobenzenes. Surprisingly, it was observed that this substrate undergoes successive multiple arylation through both C-C and C-H bond cleavages. This intriguing catalytic sequence suggests a novel reaction pathway. Overall, these findings expand our understanding of transition metal-catalyzed reactions and provide insights into the activation of inert bonds. 1
Mechanistic Insights
The palladium-catalyzed reaction of 2-hydroxy-2-methylpropiophenone involves a series of steps that lead to the formation of tetraarylethanes and isochromanones. The initial step is the R-ketol rearrangement of 2-hydroxy-2-methylpropiophenone to an intermediate compound, followed by successive catalytic diarylation to produce the desired products. This rearrangement is catalyzed by ArPd(II) species and is followed by the first arylation, leading to the formation of tetraarylethanes and isochromanones. The reverse R-ketol rearrangement of the intermediate occurs to give ketol, which then undergoes dimerization to give tetraarylethanes accompanied by the formation of diketones. The triarylation of the diketones leads to the formation of isochromanones. Additionally, the reaction of a model substrate with aryl bromides provides further insight into the mechanism, indicating the involvement of intermediary products and the participation of diarylmethylpalladium(II) species. 2
Substrate Scope
The substrate scope of the palladium-catalyzed reaction involving 2-hydroxy-2-methylpropiophenone was investigated using different aryl bromides and ligands. It was found that varying the aryl bromides and ligands led to the formation of different products with varying yields. For example, the use of p-bromotoluene and P(ptolyl)3 as a ligand resulted in the formation of 1,1,2,2-tetra(p-tolyl)ethane, while the use of P(t-Bu)3 as a ligand led to the formation of another structurally attractive compound, 1-phenyl-4,4-di(p-tolyl)7-methylisochroman-3-one. The reactions with different aryl bromides also yielded distinct products, highlighting the substrate scope of this catalytic reaction. In summary, the mechanistic insights and substrate scope of the palladium-catalyzed reaction of 2-hydroxy-2-methylpropiophenone shed light on the intricate series of steps involved in the formation of tetraarylethanes and isochromanones, as well as the range of aryl bromides and ligands that can be utilized to yield diverse products. Further studies are required to establish the detailed mechanisms and optimize the reaction conditions for broader substrate scope. 2
Reference
1. Matsumura S, Maeda Y, Nishimura T, Uemura S. Palladium-catalyzed asymmetric arylation, vinylation, and allenylation of tert-cyclobutanols via enantioselective C-C bond cleavage. J Am Chem Soc. 2003;125(29):8862-8869.
2. Wakui H, Kawasaki S, Satoh T, Miura M, Nomura M. Palladium-catalyzed reaction of 2-hydroxy-2-methylpropiophenone with aryl bromides: a unique multiple arylation via successive C-C and C-H bond cleavages. J Am Chem Soc. 2004;126(28):8658-8659.
You may like
Related articles And Qustion
Lastest Price from 2-Hydroxy-2-methylpropiophenone manufacturers

US $0.00/KG2025-04-21
- CAS:
- 7473-98-5
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $4.00/kg2025-04-21
- CAS:
- 7473-98-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100000