2-Hydroxy-2-methylpropiophenone: properties and applications
General Description
2-Hydroxy-2-methylpropiophenone (HMPP) is a colorless to pale yellow liquid with photophysical and photochemical properties that make it valuable in various applications. It has a strong absorption peak in the ultraviolet range, enabling its use in UV spectroscopy and photopolymerization reactions. HMPP can undergo Norrish type I reactions upon UV exposure, generating reactive ketyl radicals for organic synthesis. Additionally, it exhibits fluorescence behavior under UV light, allowing for detection using fluorescence spectroscopy. HMPP also acts as a photoinitiator or photosensitizer in UV-curable systems, facilitating fast curing, improved print quality, and durability in industries such as printing, coatings, and dentistry. In organic synthesis, HMPP participates in Palladium-catalyzed reactions with aryl bromides, enabling efficient carbon-carbon bond formation. Overall, HMPP's properties contribute to its wide range of applications in different fields.

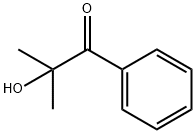
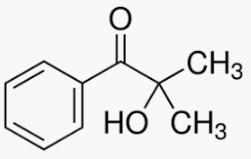
Figure 1. 2-Hydroxy-2-methylpropiophenone
Properties
2-Hydroxy-2-methylpropiophenone is a colorless to pale yellow liquid with a molecular formula of C10H12O2. It possesses intriguing photophysical and photochemical properties that make it suitable for various applications. HMPP has a strong absorption peak in the ultraviolet (UV) range, specifically around 230 nm, due to the n→π transition of its carbonyl group. This property allows it to be utilized in UV spectroscopy and photopolymerization reactions. When exposed to UV light, HMPP undergoes a Norrish type I reaction, resulting in the cleavage of the adjacent carbon-oxygen bond and the formation of a highly reactive ketyl radical and a carbonyl compound. The ketyl radical can engage in different chemical reactions, such as hydrogen abstraction and addition, which are valuable in organic synthesis. Moreover, HMPP exhibits fluorescence behavior upon photoexcitation. Under UV light, it emits fluorescence in the visible range, with a maximum emission peak at approximately 350 nm. This fluorescence characteristic enables the detection and measurement of HMPP using techniques like fluorescence spectroscopy. Furthermore, HMPP demonstrates photochemical reactivity and can act as a photoinitiator or a photosensitizer in photopolymerization reactions. When exposed to UV light alongside a suitable co-initiator or substrate, HMPP initiates or catalyzes photoinduced radical reactions, thereby initiating polymerization processes. This property finds applications in industries such as coatings, adhesives, and printing inks. Overall, the photophysical and photochemical properties of 2-Hydroxy-2-methylpropiophenone make it a valuable compound in diverse fields, including spectroscopy, organic synthesis, and photopolymerization processes. 1
Applications
Organic synthesis
2-Hydroxy-2-methylpropiophenone finds application in the Palladium-catalyzed reaction with aryl bromides. This reaction allows for the synthesis of complex organic molecules by forming carbon-carbon bonds. The reaction proceeds through a series of steps involving the coordination of the palladium catalyst to the aryl bromide, oxidative addition of HMPP to the catalyst, transmetallation, and reductive elimination. This process results in the desired product, which contains a carbon-carbon bond between HMPP and the aryl group. Moreover, this reaction can be optimized for high yields, making it suitable for large-scale applications. The mild reaction conditions and high selectivity, facilitated by the palladium catalyst, contribute to its practicality. The utility of the Palladium-catalyzed reaction with HMPP extends to areas such as pharmaceutical intermediates, natural product synthesis, and functional materials. The resulting compounds can exhibit biological activity, making them valuable for drug discovery and development. Overall, the use of HMPP in the Palladium-catalyzed reaction with aryl bromides enables efficient carbon-carbon bond formation and offers versatility in organic synthesis. This method holds promise for the creation of complex molecules with diverse applications in various scientific and industrial fields. 2
Photoinitiator
2-Hydroxy-2-methylpropiophenone is a highly efficient and versatile photoinitiator used in various UV-curable systems. It plays a crucial role in initiating and promoting polymerization reactions when exposed to UV or visible light. HMPP offers several advantages, including its strong absorption capacity, excellent solubility in organic solvents, and compatibility with different monomers and oligomers. These features enable its wide application in industries such as printing, coatings, and dentistry. HMPP provides fast curing, improved print quality, and increased productivity in UV-curable printing inks. It also offers rapid drying, high durability, and resistance to chemicals and weathering in UV-curable coatings for various surfaces. In dental materials, HMPP contributes to the formulation of light-curable composites and adhesives, offering aesthetic appeal, bonding properties, and mechanical strength. Overall, HMPP's efficiency, versatility, and control make it an indispensable component in UV-curable systems. Its use enhances product performance, increases efficiency, and improves the quality of final products in multiple industrial applications. 3
Reference
1. Chen L, Chen F, Zhu W, Chen L, Liang Y, Guo X. Construction of Photoinitiator Functionalized Spherical Nanoparticles Enabling Favorable Photoinitiating Activity and Migration Resistance for 3D Printing. Polymers (Basel), 2022, 14(21):4551.
2. Wakui H, Kawasaki S, Satoh T, Miura M, Nomura M. Palladium-catalyzed reaction of 2-hydroxy-2-methylpropiophenone with aryl bromides: a unique multiple arylation via successive C-C and C-H bond cleavages. J Am Chem Soc, 2004, 126(28):8658-8659.
3. Zhong R, Hu H, Zhou Y. Synthesis and Characterization of a Trifunctional Photoinitiator Based on Two Commercial Photoinitiators with α-Hydroxyl Ketone Structure. Materials (Basel), 2021, 14(18):5272.
Related articles And Qustion
See also
Lastest Price from 2-Hydroxy-2-methylpropiophenone manufacturers

US $0.00/KG2025-04-21
- CAS:
- 7473-98-5
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $4.00/kg2025-04-21
- CAS:
- 7473-98-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100000