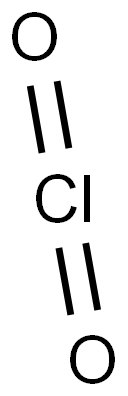Overview of ClO2 Polarity
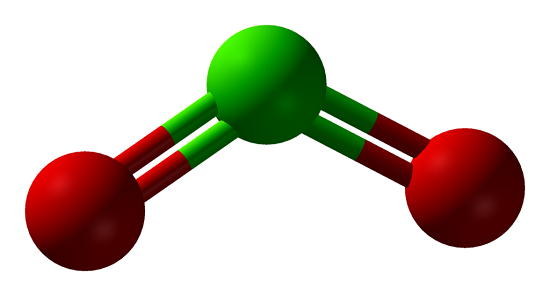
Chlorine dioxide with the molecular formula ClO2 exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and -59 °C, and as bright orange crystals below -59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach.
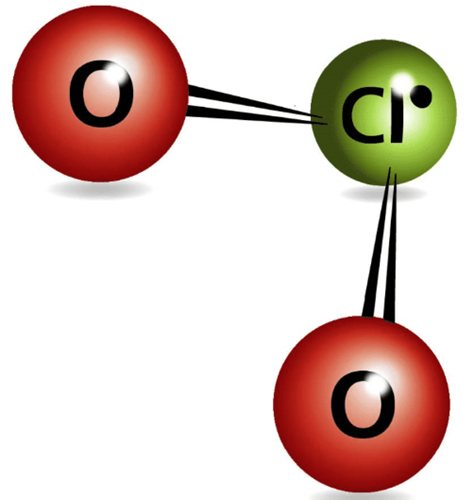
Chemical properties
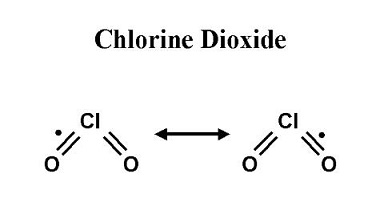
In ionic form, chlorine dioxide is known as chlorite with the molecular formula (ClO2-). It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent.
Chlorine dioxide tends to get reduced and provide oxygen to different substrates during an oxidation-reduction or redox reaction.
From this, it can be understood that chlorine dioxide in an ionic state will be a strong oxidizer of chlorine oxyanions.
Polarity of ClO2
Polar molecules interact through dipole-dipole intermolecular forces and hydrogen bonds. Polarity underlies several physical properties including surface tension, solubility, and melting and boiling points.
Uses
Chlorine dioxide is used for the bleaching of wood pulp and for the disinfection (called chlorination) of municipal drinking water, treatment of water in oil and gas applications, disinfection in the food industry, microbiological control in cooling towers, and textile bleaching. As a disinfectant, it is effective even at low concentrations because of its unique qualities.
You may like
Related articles And Qustion
See also
Lastest Price from Chlorine dioxide manufacturers

US $10.00/kg2025-06-27
- CAS:
- 10049-04-4
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON

US $50.00/kg2025-03-07
- CAS:
- 10049-04-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000