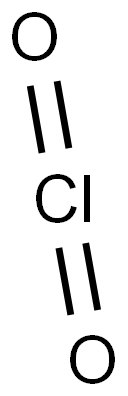Preparation Method of Chlorine dioxide
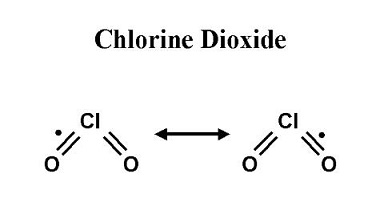
Chlorine dioxide is a yellowish-green, irritating, neutral chlorine compound with the formula ClO2[1]. CAS number is 10049-04-4. It is liquefied into reddish brown liquid at 11℃, but solidified into orange red crystal at -59℃. It decomposes into hypochlorous acid, chlorine and oxygen in hot water.When it is diluted with an inert gas such as air, carbon dioxide or nitrogen, the explosiveness is reduced. It belongs to strong oxidant with relative vapor density of 2.3g/L[2]. Chlorine dioxide can be prepared through the methods as follows:
Structure of Chlorine dioxide
Oxidation method
In this method, Cl2 or HClO is used as an oxidant to oxidize NaClO2 to form ClO2 or the oxidation and reduction reaction of NaClO2 with itself generates ClO2in an acidic medium. Most foreign water plants use this method to generate ClO2[3]. The reaction formula is as followed:
Cl2+2NaClO2→2ClO2↑+2NaCl
2NaClO2+HClO+HCl→2ClO2↑+2NaCl+H2O
Chlorine-oxidation method is characterized by less one-time investment, easy operation and easy control. The prepared ClO2 has high purity and few by-products. However, its slow response, high acid consumption, high cost, and harsh equipment conditions make it generally only suitable for laboratory and small-scale production. The high cost of NaClO2 determines the high production cost of ClO2, which is about 3 times that of chlorate method.
(1) Acidification method. Under acidic conditions, ClO2—is decomposed steadily into ClO2, ClO3- and Cl- at a measurable rate[4]. The reaction formula is as followed:
5NaClO2+4HCl→4ClO2↑+5NaCl+2H2O
Currently, ClO2 is mainly produced by hydrochloric acid or sulfuric acid/NaClO2 in acidification method. The generator technology is to let the acid (hydrochloric acid or sulfuric acid) and sodium chlorite NaClO2 solution react under air (or chlorine) flow and blow out, then the generated ClO2 is sent into the disinfection system by the ejector. The method is simple in process and convenient in operation. However, the disadvantage is that the reaction rate is slow, the acid amount is large, more waste acid is generated, and a certain amount of Cl2 is produced, which affects the purity of ClO2 and then brings trouble to the application of ClO2.
(2) Persulfate oxidation method. ClO2 is produced by using the sodium persulfate/NaClO2 system[5]. Sodium persulfate (also known as sodium persulfate)/ Na2S2O8 reacted with sodium chlorite solution to form ClO2:
2NaClO2+Na2S2O8→2ClO2↑+2Na2SO4
It is easy to prepare or generate ClO2 on the spot with tablets and powders. Due to the equivalent ratio of tablets, there is no need to measure the dose. In the actual treatment process, as long as a certain amount of tablets is dissolved in a certain volume of water, a certain concentration of ClO2 solution can be obtained for use in the disinfection system.
2. Electrolysis method
The electrolysis method uses sodium chlorate or sodium chloride as raw materials to prepare ClO2 by the diaphragm electrolysis technique. The electrolyte used may be salt solution, chlorite solution or chlorate solution. In the electrolysis process, a caustic soda solution and hydrogen are produced at the cathode, and the mixture of ClO2, chlorine gas, hydrogen peroxide and ozone is obtained at the anode[6].
Chlorine dioxide is toxic and therefore needs to be restricted to ensure its safe use. The US Environmental Protection Agency has set the maximum level of chlorine dioxide in drinking water to 0.8 mg/L. The Occupational Safety and Health Administration (OSHA) is an agency of the US Department of Labor that uses chlorine dioxide. The person set an allowable exposure limit of 0.1 ppm (0.3 mg/m3) for 8 hours.
References
[1] https://en.wikipedia.org/wiki/Chlorine_dioxide
[2] Annex 4 Immediately Dangerous to Life and Health concentrations (IDLH)[J]. Industrial Safety, 2008:345–346.
[3] Ina T, Miura H, Nakajima H, et al. Oxidation method:, EP 1074536 B1[P]. 2009.
[4] Zabar S, Lesmes U, Katz I, et al. Structural characterization of amylose-long chain fatty acid complexes produced via the acidification method.[J]. Food Hydrocolloids, 2010, 24(4):347-357.
[5] Yang R, Zhao M X, Zhou J B. Effects of different conditions on the different of total nitrogen in solution by persulfate oxidation method[J]. Journal of Northwest Sci-Tech University of Agriculture and Forestry, 2005.
You may like
Related articles And Qustion
See also
Lastest Price from Chlorine dioxide manufacturers

US $10.00/kg2025-06-27
- CAS:
- 10049-04-4
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON

US $50.00/kg2025-03-07
- CAS:
- 10049-04-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000