Synthesis of Belantamab Mafodotin
Synthesis of Belantamab Mafodotin
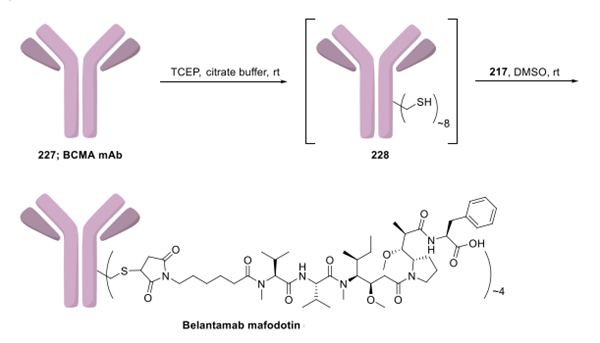
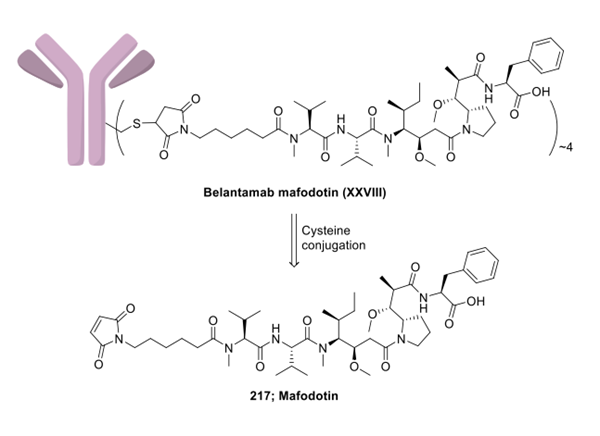
Belantamab Mafodotin is prepared by combining mafodotin with the monoclonal antibody belantamab. A drug linker technology was used to bind mafodotin (217) to monoclonal antibody belantamab. Linker-MMAF intermediate, mafododine. The method for the assembly of the leucovorinumab mafododine payload connector is shown below:
Step 1: Prepration of Mafodotin
The route described below has been chosen due to clarity and completeness of the experimental procedure. Synthesis of mafodotin (217) commenced with the protection of Fmoc-L-phenylalanine (218) as 2,4- dimethoxybenzyl (DMB) ester followed by Fmoc deprotection to generate free amine 220. Phenylalanine-ODMB 220 was then coupled with N-Fmoc-proline derivative 222 to afford amide 223. The Fmoc protecting group within 223 wasremoved by the treatment of dimethylamine in DCM. The free amine participated in a TBTU-mediated amide formation reaction with carboxylic acid 224 to deliver bis-protected MMAF 225. Selective deprotection of Fmoc on 225 exposed a free amine that was coupled with linker 226 under amide bond formation conditions. The DMB protecting group was then removed with TFA in DCM to complete the synthesis of linker-payload construct mafodotin (217).
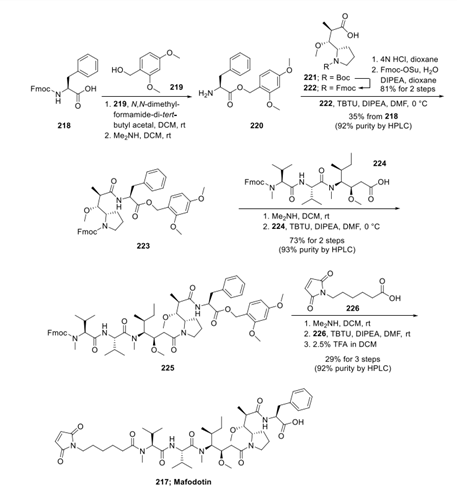
The synthesis of N-Boc-dolaproine 221 has been described in an earlier review toward a related ADC, brentuximab vedotin. N-Boc-dolaproine 221 was converted to N-Fmoc-dolaproine 222 using a standard deprotection−reprotection protocol. Although the preparation of 221 and carboxylic acid fragment 224 has been described previously, these subunits are currently commercially available from multiple vendors.
Step 2: Prepration of Belantamab Mafodotin
Preparation of the final drug product via conjugation of mafodotin with mAb belantamab is described. Although a detailed experimental procedure was not disclosed, authors revealed implementation of their own "TCEP protocol" for its preparation. First, belantamab mAb 227 was treated with tris(2-carboxyethyl)phosphine (TCEP) in the presence of citrate buffer (25 mM, pH ∼ 7.4) at room temperature to expose approximately eight sulfhydryl moieties (228). The free sulfhydryls reacted with mafodotin (217) in DMSO to provide drug product belantamab mafodotin. The authors did not comment on subsequent oxidation and quenching of any nonreacted sulfhydryl groups.