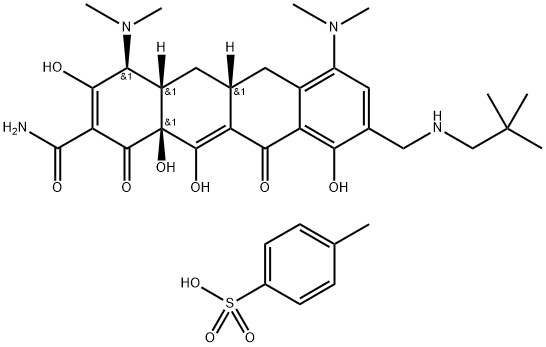
OMadacycline (Tosylate): A Promising Tetracycline Antibiotic for the Treatment of Bacterial Infections
General Description
OMadacycline (tosylate) is a tetracycline antibiotic that inhibits protein synthesis in bacteria by binding to the 16S rRNA component of the 30S ribosomal subunit. Its competitive inhibition is reversible and primarily occurs at the Tet1 binding site on the 30S subunit. OMadacycline (tosylate) has a higher affinity for the 30S ribosome than tetracycline and is effective against tetracycline-resistant strains due to its relative insensitivity to efflux pumps and ribosomal protection proteins. The pharmacokinetics of OMadacycline (tosylate) show a mean volume of distribution of 168-204 L, with a terminal half-life of 11.4-17.1 hours. Its activity is associated with the PD index known as free AUC divided by the MIC (fAUC 24h/MIC). Studies demonstrate the pharmacodynamic properties of OMadacycline (tosylate), its relationship with MIC values, and its potential efficacy in treating bacterial infections.

Figure 1. Tablets of OMadacycline (tosylate)
Mechanism of Action
OMadacycline (tosylate) is a tetracycline antibiotic that exerts its bacteriostatic activity by inhibiting protein synthesis in bacteria. It binds to the 16S rRNA component of the 30S ribosomal subunit, effectively blocking the access of aminoacyl-tRNA to the A-site of the ribosome. This competitive inhibition is reversible and primarily occurs at the Tet1 binding site on the 30S subunit. Divalent ions such as magnesium are crucial for the transport and binding of tetracyclines to their targets. Once inside the bacterial cell, OMadacycline (tosylate) dissociates from magnesium and diffuses across the cytoplasmic membrane via passive diffusion. OMadacycline (tosylate) has a higher affinity for the 30S ribosome than tetracycline and is effective against tetracycline-resistant strains due to its relative insensitivity to efflux pumps and ribosomal protection proteins. The mechanism of action of OMadacycline (tosylate) is similar to other tetracycline antibiotics such as minocycline, doxycycline, tigecycline, and eravacycline. However, OMadacycline (tosylate) has been reported to be more efficient than tetracycline and has a lower IC50 value against bacteria such as S. aureus. 1
Pharmacokinetics
OMadacycline (tosylate) is a tetracycline-class antibiotic that has been studied extensively for its pharmacokinetics both orally and intravenously. The oral bioavailability of OMadacycline (tosylate) was found to be 34.5% in fasted individuals, with similar results from different formulations. However, food intake, especially dairy-containing food, significantly affected absorption. Most of the drug was eliminated unchanged in feces, with only a small percentage excreted in urine. Intravenous administration showed a mean volume of distribution of 168-204 L, with a terminal half-life of 11.4-17.1 hours. Approximately 20% of the intravenous dose was excreted unchanged in urine over 24 hours. OMadacycline (tosylate) did not induce or inhibit major cytochrome P450 enzymes and had low protein binding compared to other tetracyclines. Intrapulmonary penetration was characterized by a ratio of AUC values in epithelial lining fluid and alveolar cells to the free AUC in plasma. The PK of OMadacycline (tosylate) was also studied in patients with renal and hepatic impairment, and no dosage adjustments were recommended. Comorbidities and other factors did not significantly affect total drug clearance. These findings provide important information for clinicians in prescribing OMadacycline (tosylate) in the treatment of bacterial infections. 2
Pharmacodynamics
In the case of OMadacycline (tosylate), a tetracycline class antibiotic, its activity is associated with the PD index known as free AUC divided by the MIC (fAUC 24h/MIC). Studies conducted by Lepak et al examined the in vivo activity of OMadacycline (tosylate) against different strains of bacteria. In a pneumonia model using neutropenic mice, OMadacycline (tosylate) doses were administered and measured in plasma and ELF (extracellular fluid). The study found that plasma AUC 24h/MIC correlated with antibacterial activity, but there was inter-isolate variability not explained by OMadacycline (tosylate) MIC or penicillin resistance. In another study using a thigh infection model in mice, the antibacterial activity of OMadacycline (tosylate) was again associated with total plasma AUC 24h/MIC. No significant differences in PD thresholds were observed between MSSA and MRSA isolates. Monte Carlo simulations were used to predict the probability of target attainment in patients with ABSSSI (acute bacterial skin and skin structure infections). Three dosing regimens of OMadacycline (tosylate) were investigated, and the simulations showed high predicted target attainment for bacteriostasis with all three regimens. Bhavnani et al studied the clinical PD of OMadacycline (tosylate) in the treatment of ABSSSI using data from phase III studies. Logistic regression analysis was used to describe the PD relationship between fAUC 24h/MIC and early clinical response. Monte Carlo simulations predicted high early clinical success rates with the tested dosing regimens. Overall, these findings demonstrate the pharmacodynamic properties of OMadacycline (tosylate), its relationship with MIC values, and its potential efficacy in treating bacterial infections. 2
Reference
1. Draper MP, Weir S, Macone A, et al. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother. 2014;58(3):1279-1283.
2. Zhanel GG, Esquivel J, Zelenitsky S, et al. Omadacycline: A Novel Oral and Intravenous Aminomethylcycline Antibiotic Agent. Drugs. 2020;80(3):285-313.
Related articles And Qustion
Lastest Price from OMadacycline (tosylate) manufacturers
US $0.00/kg2025-06-13
- CAS:
- 1075240-43-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg
US $0.00-0.00/kg2025-04-16
- CAS:
- 1075240-43-5
- Min. Order:
- 5kg
- Purity:
- 98%-102%
- Supply Ability:
- 200kg