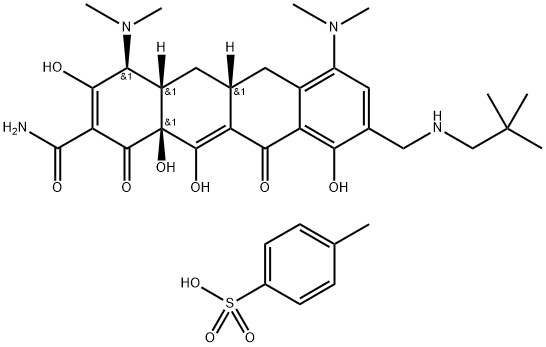
Description
Omadacycline tosylate
is a semisynthetic tetracycline-class
antibacterial agent, which has been
designated by some as a “new-generation tetracycline” or “the first in a new
class of aminomethylcyclines.” Omadacycline has been structurally
modified to provide effectiveness against
certain strains of bacteria that have
mechanisms to resist the action of older
tetracyclines. The new agent binds to the
30S ribosomal unit, blocks bacterial
protein synthesis, and is generally
bacteriostatic
[1]. Omadacycline tosylate has broad-spectrum antibacterial activity against aerobic and anaerobic Gram-positive and Gram-negative bacteria, as well as atypical bacteria. Omadacycline tosylate has been studied in acute bacterial skin and skin structure infections, community-acquired pneumonia and urinary tract infections.
Uses
OMadacycline (tosylate) is a new type of 9-aminomethylcycline drug, which is a semi-synthetic compound obtained by modifying chemical groups on the basis of minocycline. The modification can help omacycline overcome bacterial resistance. Pharmacological properties, expand the antibacterial spectrum, and improve the pharmacokinetic properties.
Biological Activity
OMadacycline (tosylate) is an aminecycline antibiotic with antibacterial activity against Gram-positive and negative aerobic bacteria and some anaerobic bacteria.
Mode of action
(1) OMadacycline (tosylate) specifically binds to the A site of the 30S subunit of the bacterial ribosome, inhibits the normal binding of aminoacyl-tRNA to this site, terminates the elongation of the peptide chain, and blocks the synthesis of proteins to produce a bacteriostatic effect.
(2) The electron-donating group (dimethylamino) of the modified group of it can improve the activity and help to overcome the bacterial efflux pump resistance mechanism.
(3) The aminomethyl modification of it can make it overcome the bacterial ribosome protection mechanism (caused by the ribosome protection protein gene TetO) and enhance oral bioavailability.
(4) But it cannot resist the effect of Tet(X) modification enzyme.
References
[1] DANIEL A. HUSSAR; Elias B C. Omadacycline tosylate, Sarecycline hydrochloride, Rifamycin sodium, and Moxidectin[J]. Journal of the American Pharmacists Association, 2019. DOI:10.1016/j.japh.2019.07.016.