Nitrogen: importance and Lewis structure
The chemical properties of Nitrogen
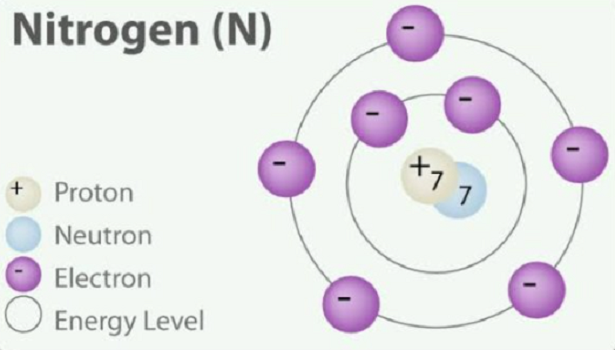
Nitrogen (N2) is the periodic table's first member in Group 15 (VA). Nitrogen is in a family group named after itself. Other elements in the nitrogen family are phosphorus, arsenic, antimony, and bismuth. Nitrogen is one of the most interesting of all chemical elements. It is a colorless, odorless, tasteless gas that is the most plentiful element in Earth's atmosphere and constituent of all living matter. Nitrogen is an essential macronutrient required for plant growth and development and significantly impacts crop yield and biomass. However, excessive application of N-based fertilizer results in environmental pollution and increases cultivation costs[1].
The element exists as N2 molecules for which the bond energy of 226 kilocalories per mole is exceeded only by carbon monoxide, 256 kilocalories per mole. Because of this high bond energy, the activation energy for the reaction of molecular nitrogen is usually very high, causing nitrogen to be relatively inert to most reagents under ordinary conditions.
A nitrogen atom has an electronic structure represented by 1s22s22p3. The five outer shell electrons screen the nuclear charge quite poorly, showing that the effective nuclear charge felt at the covalent radius distance is relatively high. Thus, nitrogen atoms are relatively small in size and high in electronegativity, being intermediate between carbon and oxygen in both of these properties.
Lewis structure
The Lewis structure of N2 is shown below:

The N2 Lewis structure is crucial because it helps us understand nitrogen molecules' molecular geometry and chemical bonding. By examining the Lewis structure, we can determine the number of bonding pairs and lone pairs of electrons in the molecule. In the case of N2, each nitrogen atom contributes 3 valence electrons to form a triple bond between them. This leaves 2 valence electrons on each nitrogen atom, represented as Lone pair electrons. The Lewis structure of N2 can be visualized as N≡N, with each nitrogen atom having one lone pair.
In the case of N2, the triple bond between the nitrogen atoms contributes to the molecule's stability. The sharing of electrons in the covalent bonds allows each nitrogen atom to achieve a stable octet, satisfying the octet rule. This stable electron configuration makes nitrogen molecules relatively inert and less reactive than other elements.
Reference
[1] SeoJun Sung, KimJu-Kon. "Nitrogen molecular sensors and their use for screening mutants involved in nitrogen use efficiency." Plant Science 298 (2020): 110587.