Does nitrogen have a 2 charge?
Nitrogen is a chemical element with the symbol N and atomic number 7. Nitrogen is a non-metallic element in the second row and 15th group. Nitrogen is a gas at room temperature.
Nitrogen appears as a colorless, odorless gas. Noncombustible and nontoxic. It makes up the major portion of the atmosphere but will not support life by itself. Nitrogen is the most common gas in our atmosphere, and it makes up over 78% of the atmosphere. Used in food processing, purging air conditioning and refrigeration systems, and pressuring aircraft tires. It may cause asphyxiation by displacement of air.
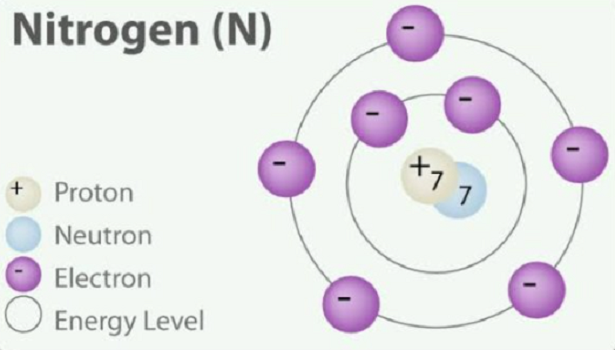
If we are talking about the monatomic element nitrogen, it has five valence electrons. The charge on an atom is related to its valence electrons or oxidation state. An atom of an element is most stable when its outer electron shell is filled or half-filled. The most common charges are based on maximum stability for the atom. However, other charges are possible. The charge of the N atom is 3-.
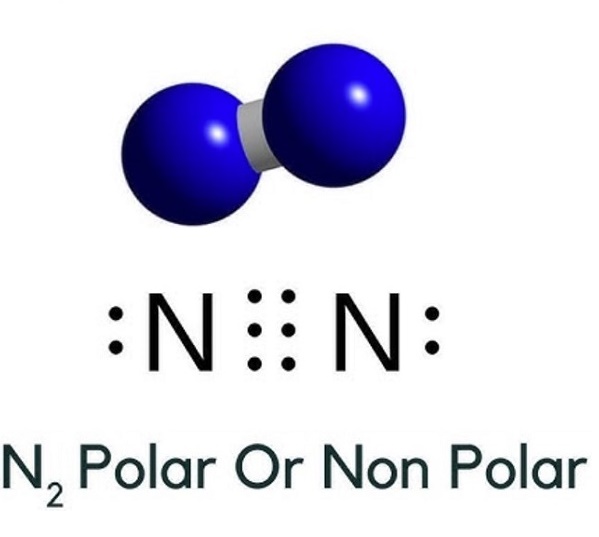
The charge of Nitrogen (N2) is 0.
The nitrogen atom shares three bonding pairs, has one lone pair, and has 5 valence electrons.
Formal Charge = (# of valence electrons in free atom) - (# of lone-pair electrons) - (1/2 # of bond pair electrons)
The formal charge on the nitrogen atom is therefore
Formal Charge of N = (5 valence e-) - (2 lone pair e-) - (1/2 x 6 bond pair e-) = 0
In short, charge of N = 5 - (2 + 6/2) = 0.